► Click here to download the original poster (PDF)
K.Hickey and S.Kitchen, Sheffield Haemophilia and Thrombosis Centre, Sheffield, UK
The laboratory classification of lupus anticoagulant (LA) is via prolongation of phospholipid-dependent clotting tests. Guidelines currently recommend the use of two screening assays. The first test should be dilute Russell's viper venom time (dRVVT) and the second should be a LA-sensitive APTT1,2. Xa-DOAC interference in DRVVT is a growing issue. Different reagents have varying sensitivity to Xa-DOAC with risk of false positive or negative depending on reagents3.
To evaluate the performance of LA screening tests tested on the Yumizen G1500 (HORIBA, France) against predicate LA screening tests tested on the Sysmex CN6000 (Sysmex, UK)
Xa-DOAC Spiking
Yumizen G APTT, Yumizen G APTT Liq and DVVtest/DVVconfirm demonstrated good repeatability/reproducibility in both normal (CV <2.5% and <8.9%) and abnormal (CV <5.2% and <11.1%) samples (see Table 1).
LA Negative Group: DVVtest/DVVconfirm identified 46/48 samples as LA negative ( ratio <1.27). Two samples gave abnormal NSCR (1.41 and 1.47). Prolongation APTT was seen in Yumizen G APTT (n=12), Yumizen G APTT Liq (n=10), Actin FS (n=6) and Actin FSL (n=10).
LA Positive Group: DVVtest/DVVconfirm identified 25/41 samples as LA positive (ratio >1.27). Prolongation APTT was seen in Yumizen G APTT (n=24), Yumizen G APTT Liq (n=18) , Actin FS (n=5) and Actin FSL (n=21).
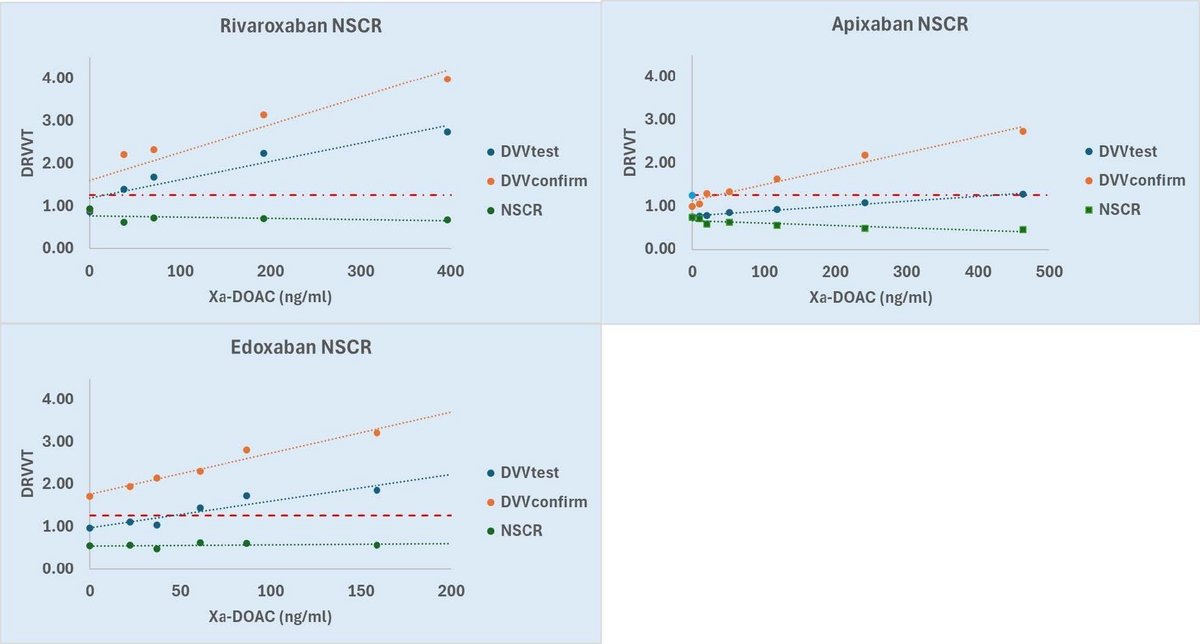
DVV test/DVV confirm showed different sensitivity to Xa-DOAC. For all Xa-DOAC spiking, NSCR remained below positivity cut off as concentration increased (Figure 1).
| Test | Sample type | Repeatability | Reproducibility | ||||
| n | Mean (sec) | CV (%) | n | Mean (sec) | CV (%) | ||
| APTT | Normal | 10 | 33.1 | 1.7 | 20 | 37.3 | 8.9 |
| APTT | Abnormal | 10 | 64.3 | 2.4 | 17 | 71.3 | 9.9 |
| APTT Liq | Normal | 10 | 29.9 | 2.5 | 23 | 35 | 7.2 |
| APTT Liq | Abnormal | 10 | 66.6 | 3.7 | 19 | 67 | 11.1 |
| DVVtest | Normal | 10 | 37.3 | 1.1 | 21 | 39.1 | 8.1 |
| DVVtest | Abnormal | 10 | 60.1 | 1.2 | 22 | 81.1 | 9.1 |
| DVVconfirm | Normal | 10 | 28.2 | 2.2 | 20 | 32.5 | 8.5 |
| DVVconfirm | Abnormal | 10 | 32.7 | 5.2 | 19 | 37.9 | 9.6 |
Table 1. Performance Characteristics of Yumizen G APTT, Yumizen G APTT Liq, DVVtest, and DVVconfirm
Figure 1: Dose-response curves for DRVVT vs Xa-DOAC concentration Dose-response curves of the normalised DVVtest ratio, normalised DVVconfirm ratio and normalised screen/confirm ratio (NSCR)
Sie haben Fragen oder Wünsche? Nutzen Sie dieses Formular, um mit unseren Spezialisten in Kontakt zu treten.