Measurement of Dissolved Oxygen Levels by the Diaphragm Electrode Method

In principle, measurement of dissolved oxygen levels requires calibration and stirring the sample water during measurement, and measurements are affected by the salt concentration, temperature, and atmospheric pressure.
DO Standard Solution Used for Sensitivity Calibration
In principle, the following solutions are used for sensitivity calibration in the diaphragm electrode method:
(1) Solution containing no DO (pure water to which an excessive amount of sodium sulfite has been added)
(2) DO air saturated solution (pure water bubbled with air)
(3) DO pure oxygen saturated solution (pure water bubbled with pure oxygen)
Since the output from a DO sensor used in the diaphragm electrode method is proportional to the partial pressure of oxygen, pure water exposed to the atmosphere for a certain period of time (about 2 to 3 minutes) can be used for calibration instead of solution 2 above. Our water quality checkers, which are often used outside, use a DO measurement device based on this calibration method.*
The solution containing no DO (solution 1) is used for calibration as the excessive amount of sodium sulfite reacts with DO and completely removes it. Prepare the air saturated solution (solution 2) by bubbling pure water with air in a small container using a small pump (aquarium pumps, etc. may be used) for two to ten minutes. Prepare the pure oxygen saturated solution (solution 3) by bubbling pure water in a small container with a pure oxygen cylinder for two to ten minutes. When bubbling with pure oxygen, do not allow fire in the area.
Generally, solution 1 is used for zero calibration, and solution 2 or pure water exposed to the atmosphere is used for DO saturation calibration. In principle, calibration and measurement of DO in a sample solution should be performed at a constant temperature (for example, at 25ºC).
* HORIBA, Ltd. (inventors: Takeshi Mori, Hiromi Okawa, and Satoshi Kono), Japanese Published Examined Application No. 7-113630 (The application was filed in 1992.)
Stirring Sample Water
In order to allow DO in a sample solution to come in contact with the diaphragm of the DO sensor at a constant rate, the sample solution must be stirred at a constant rate. In order to do this, a flow cell is sometimes used.
For measurement in a laboratory, use a magnetic stirrer to stir the sample solution at a constant rate (at a rotation speed that does not form an eddy (500 to 1,000 rpm)). If use of a stirrer causes an increase in sample temperature, use a constant-temperature bath. For measurement in a field site, measure the DO level while moving the electrodes up and down at a constant speed (about 30 cm every 2 seconds).
Influence of Salt Concentrations
As mentioned above, the saturation level of dissolved oxygen is affected by the concentration of salt contained in the sample water, and the higher the concentration of salt is, the lower the saturation level of DO is.
This can be seen clearly in Figure 1, which shows the relationship between the concentration of chloride ions (Cl-) and the saturation level of dissolved oxygen. However, the output of the diaphragm electrode method, whether it is the diaphragm galvanic electrode method or the diaphragm polarographic method (the term “diaphragm electrode method” refers to both methods) corresponds to the partial pressure of oxygen rather than the level of dissolved oxygen, and does not reflect the influence of the salt concentration. This means that you can calculate the salt concentration of the sample solution and correct (or reduce) the DO level accordingly based on the calculated value.
Salt concentrations can be calculated from conductivity measurements. We sell a model that incorporates a function that uses this system to correct DO levels according to salt concentrations. The rates of reduction of DO levels for different concentrations of salt in sample solutions are listed in the rightmost column “Amount of DO to be deducted per 100 mg/L of chloride ions (Cl-)” in Table 1 on the page “What Is Dissolved Oxygen?”
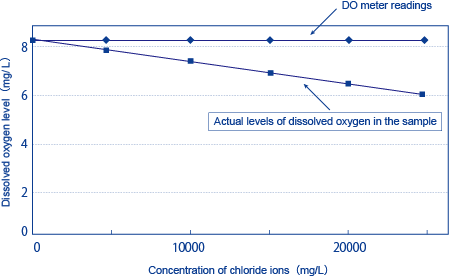
Figure 1: Chloride Ion Concentration and Dissolved Oxygen Saturation at 25?
Influences of Temperature and Atmospheric Pressure
The influence of temperature on the DO sensor for the diaphragm electrode method mainly comes from changes in the rate at which DO passes through the diaphragm. The higher the temperature becomes, the higher the rate at which DO passes through the diaphragm becomes and the higher the sensitivity of the DO sensor becomes. The influence of temperature on saturation levels of DO is as shown in Table 1 on the page “What Is Dissolved Oxygen?” Aside from this influence, the influence of temperature on the DO sensor is considered here.
Figure 2 shows the influence of temperature on the output of the DO sensor of our Multiparameter Water Quality Checker (model: U-50) (diaphragm polarographic method).
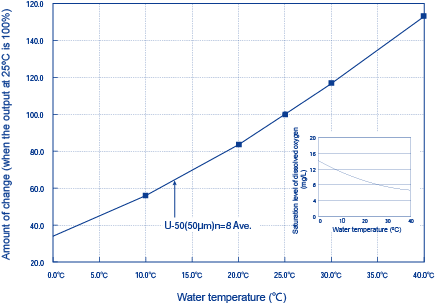
Figure 2: The influence of temperature on DO sensor output (Diaphragm Electrode Method)
In addition, the diaphragm electrode method measures the partial pressure of oxygen. Thus, the output is changed in proportion to the atmospheric pressure. For example, while the atmospheric pressure at sea level is 1 atm (1013 hPa), at 5,000 m above sea level it is reduced to about 0.5 atm. At this height, the output of the DO sensor is reduced by about half, compared to the output at 1 atm. When comparing various kinds of DO data, whether or not it has been corrected for atmospheric pressure must be checked. For example, if the DO level measured at 25ºC and an atmospheric pressure of 980 hPa is 6.5 mg/L, the DO level can be corrected to the value at 25ºC and 1013 hPa (1 atm) as follows:
![]()
Based on this idea, an instrument can be produced that combines a DO sensor for the diaphragm electrode method with a barometer and shows values after correction for atmospheric pressure (values at 1 atm).*
The deeper water becomes, the higher the water pressure becomes. Atmospheric pressure increases by about 1 atm for every 10 m of water depth. The readings of a DO sensor can be corrected to values at 1 atm based on the idea of water pressure detection for water depth measurement.*
* HORIBA, Ltd. (inventor: Takeshi Kobayashi), Patent No. 3959166 (The application was filed in 1997.)