Measurement of calcium in milk and milk beverages with LAQUAtwin
The amount of calcium in milk and milk beverages is generally determined using atomic absorption spectroscopy (AA) or inductively coupled plasma atomic emission spectroscopy (ICP). Ion chromatography (IC) using acidic elute can also measure total calcium amount regardless of the form in which the calcium exists. Alternatively, the calcium ion selective electrode method measures the amount of free calcium ions. In addition, chelate titration allows measurement of free calcium ions and part of the calcium bound to other substances.
However, by ionizing protein-bound calcium using an acidizing pretreatment,, the calcium ion-selective electrode method (LAQUAtwin Ca2+) can be used to measure the total amount of calcium.
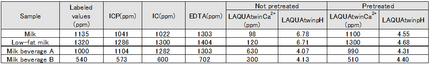
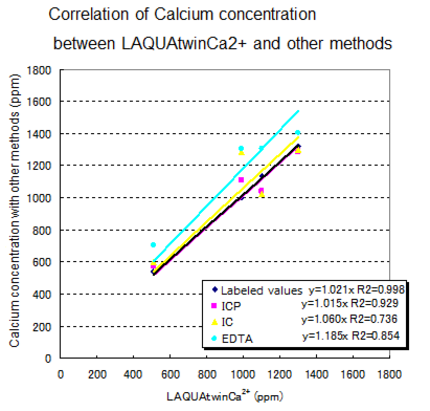
The following gives an explanation of this based on empirical data—measured by a variety of methods—on the calcium content of milk and milk beverages.For each type of sample, the following table shows the labeled value, together with the values measured by various methods. The diagram below shows the correlation between LAQUAtwin Ca2+ data and that from other methods for a pre-treated sample. Without the need for a large measurement system, LAQUAtwin Ca2+ produced values of total calcium amount that are approximately equal to those obtained by other methods.
Pretreatment of the lactic drink sample consisted of adjustment to a pH of around 2, followed by readjustment to a pH of 4 prior to measurement being carried out.
Pretreatment procedure (for milk)
- Place 5 mL of sample solution in a 100 mL beaker.
- In the case of milk, add 50 to 60 μL of 5M hydrochloric acid to the sample in step 1.
- Use LAQUAtwin to confirm that the sample has a pH of 4.3 to 4.6. Return the sample solution to the beaker.
- Add 45 mL of water to the sample created in step 2, thereby diluting it tenfold.
- Wait for a few minutes to allow the protein to precipitate then collect a sample of the supernatant solution.
Pretreatment procedure (for milk beverages such as lactic drinks)
- Place 5 mL of sample solution in a 100 mL beaker.
- Add 100 μL of 5M hydrochloric acid to the sample and confirm that it has a pH of around 2 using LAQUAtwin. Return the sample solution to the beaker.
- Add 45 mL of water to the sample created in step 2, thereby diluting it tenfold.
- Add approximately 0.05 g of tris-hydroxy-aminomethane to the solution and confirm that its pH has changed to around 4.3 to 4.6 using LAQUAtwin pH.
- Wait for a few minutes to allow the protein to precipitate (sediments may not be visible to the naked eye) then collect a sample of the supernatant solution.
Measurement procedure

As a supporting electrolyte, add 0.0375 g of potassium chloride to 5 mL of the supernatant liquid from the pre-treated sample solution, and dissolve by stirring.
Measure the sample with a LAQUAtwin Ca2+ that has been calibrated against reference solution, and use the dilution ratio to calculate the result.
Next Page Measurement of potassium in various soils with LAQUAtwin