HORIBA Medical has introduced more pre-analytical functionality to its Yumizen G800 hemostasis analyzer with the launch of its latest software version 2.36. It is well recognised that pre-analytical conditions are critical in hemostasis and generate up to 70% of the Hemostasis results errors in a laboratory.*1*2 The new Tube Filling Level Check option for the Yumizen G800 helps to overcome a significant pre-analytical error risk factor.
The correct filling of the sample tube is one of the most sensitive pre-analytical parameters in blood coagulation analysis. If filled inaccurately, this will have a direct impact on results accuracy as it changes the ratio of anti-coagulant (normally 1 : 9). Therefore, the tube filling tolerance is clearly indicated on a sample tube to support manual operator checking.
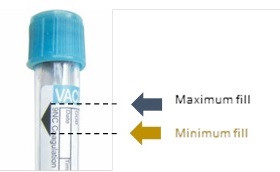
In case of incorrect manual checking of tube fill volume, the new Yumizen G800 software version 2.36 now integrates an optional automatic check of tube filling (using a level detection sensor) that triggers an alarm in the incidence of level abnormality which will directly affect final results.
An insufficient fill volume greatly modifies the fixed blood-to-anticoagulant ratio and increases the dilution of the sample due to the volume of liquid anticoagulant. This may increase the clotting time due to the excess calcium-binding citrate present. It has been reported in literature that when tubes are drawn at less than 89% of total fill, a clinically significant bias exists in test results for APTT, less than 78% for fibrinogen, and less than 67% for coagulation factor VIII.*2
Further supporting hemostasis results accuracy, HORIBA Medical has also published the HORIBA Medical Hemostasis Pre-analytical Guidelines, which are based on CLSI and GFHT recommendations. These detail all key information on pre-analytical conditions and their potential impact on final results, including the filling volume of the sample tube.
In addition, a HORIBA Medical Hemostasis Guide is also available. This handy pocket-sized Hemostasis Guide is published in collaboration with Dr. Pierre Suchon, University Hospital La Timone, APHM (Assistance Publique - Hopitaux de Marseille). The Guide introduces the basic principles of hemostasis and has been created particularly for students, laboratory staff, trainee medics, and anyone else with an interest in hemostasis and thrombosis. It covers the principle tests carried out in hemostasis labs, as well as relevant and useful information that reinforces quality and safety criteria applicable to this area.
The HORIBA Medical Hemostasis Pre-analytical Guidelines and HORIBA Medical Hemostasis Guide are available to download at: https://www.horiba.com/en_en/medical/products/hemostasis/
References:
*1: Lippi G, Banfi G, Buttarello M et al. Recommendations for detection and management of unsuitable samples in clinical laboratories. Clin Chem Lab Med. 2007 ; 45(6):728-736.
*2: Lippi G, Salvagno GL, Montagnana M, Lima-Oliveira G, Guidi GC, Favaloro EJ. Quality standards for sample collection in coagulation testing. Semin Thromb Hemost. 2012;38:565–75.
