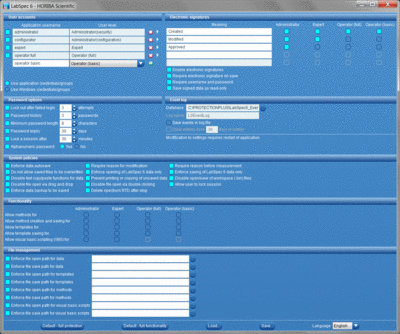
User account management and access control
Full control allowing only authorized users to access LabSpec 6, based on either a fully integrated user account module, or via re-authentication of a user’s Windows® credentials. Each user can be assigned to a specific user level, each offering different levels of functionality to match the user’s experience and measurement requirements.
Audit trail
Each data file incorporates a full file history, tracking all data acquisition, modification and electronic signing events, with user details and time stamp. Additional, key LabSpec 6 operations are recorded in an external event log, including events such as software start/stop, user log in attempts, and file operations (open, save, modify).
Electronic signatures
Users can digitally authenticate their data with legally binding electronic signatures, which are applied and attached directly to the electronic record (data). Different meanings can be associated to the signings, with different user levels accessing different meanings.
System policies and file management tools
A range of optional policies can be applied, allowing administrators to heighten security and data within LabSpec 6. Additionally, data archiving can be facilitated by enforcing specific data save pathways, ensuring that all data is saved into secure archived locations.
HORIBA Scientific also offers qualification protocols for its Raman systems, for both installation (IQ) and operation (OQ). These ensure that system validity can be demonstrated, a requirement of the 21 CFR part 11 regulations.
Further information:
Detailed document about HORIBA Scientific’s compliance to 21 CFR Part 11 with its LabSpec 6 ProtectionPlus software is available. Please request the document 21CFR11 - HORIBA response for LabSpec 6 with ProtectionPlus.