► Click here to download the full text file (PDF).
The invention of anti-microbial treatments represented a major step forward in human health, protecting us against a host of diseases – from the deadly malarial parasite and the virus responsible for COVID-19, through to the most common fungal and bacterial infections. This is especially true in the case of antibiotics, without which even the most seemingly innocuous wounds can become lethal - the advent of antibiotics has saved countless lives over the past decades. However, bacteria are now fighting back rendering certain antibiotics ineffective…what do we have left in our armoury to counter this serious global threat?
Consider the famous anecdote of Albert Alexander, a constable in the Oxford police force. In December 1940, he suffered a minor scratch on the mouth from a rose thorn, and yet a month later he was hospitalised with a severe case of septicaemia.
With the effects of penicillin in clearing bacterial infections having first been noted by Sir Alexander Fleming in 1928, its full promise was only just being realised when the case of Albert Alexander came to the attention of Howard Florey and his team of researchers at Oxford University. Having just discovered the capabilities of combating deadly bacterial infections in rodents, the team now chose their first human patient.
Despite a positive and rapid response, the technology at the time severely restricted antibiotic purification, and the supplies were simply not enough to save Albert Alexander, who relapsed and died of his injuries the following spring. An unfortunate story in itself, yet this signalled the beginning of the antibiotic era that we have enjoyed for many decades since.
However, bacteria have now responded to strong selection pressures and, with the widespread use of antibiotics having spawned a new generation of antibiotic-resistant strains (aka ‘superbugs’), the power of antibiotics is waning. Treatments for common infections are fast running out and antimicrobial resistance (AMR) presents a major health issue globally, with a high proportion of resistance reported within both the healthcare setting and the general community. Without appropriate and immediate action, routine surgery and many immune-suppressing cancer treatments may soon become simply too dangerous due to the risk of infection.
AMR has been described as a silent pandemic. It is estimated that 700,000 people around the world each year die from a drug-resistant infection, and that this number will rapidly climb to 10 million a year by 2050 unless action is taken to slow the spread [1].
On the molecular level, AMR is essentially a natural phenomenon where bacteria respond to their environment just like any organism, mutating and evolving to protect themselves against the toxic compounds to which they are exposed. The number of bloodstream infections has increased by 17% between 2015 and 2019, with a 32% increase in the estimated number of antibiotic resistant bloodstream infections. This is primarily driven by the Gram-negative bacterial species Escherichia coli and Klebsiella pneumoniae and the Gram-positive bacterial genera Enterococcus spp. (Figure 1) [2]. Reported cases of antibiotic-resistant bacterial strains are increasing year on year (Figure 2) [2].
Figure 1. Source (ESPAUR report 2019-2020) [2]
* key pathogens include: E. coli, K. pneumoniae, K. oxytoca, Acinetobacter spp. Pseudomonas spp., Enterococcus spp., S. aureus and S. pneumoniae.
+ E. coli, K pneumoniae and K. oxytoca: resistant to any of: carbapenems, third-generation cephalosporin, aminoglycosides or fluoroauinoquinolones; Acinetobacter spp: resistant to aminoglycosides and fluoroquinolones, or carbapenems; Pseudomonas spp. resistant to three or more antimicrobial groups, or carbapenems; Enterococcus spp. resistant to glycopeptides; S. aureus resistant to meticillin; S. pneumoniae resistant to penicillin and macrolides, or penicillin.
Figure 2. Source (ESPAUR report 2019-2020) [2]
Providing a highly insightful view of the situation within the UK, the latest 2021 report by the UK Health Security Agency highlights how antibiotic prescribing and resistance throughout the UK has changed over recent years [3]. The English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) report shows that although AMR is on the increase, antibiotic prescribing throughout the UK has decreased since its peak in 2014.
The Global Antimicrobial Resistance and Use Surveillance System (GLASS) published by WHO recently reported high rates of resistance against antibiotics used to treat common bacterial infections on a global scale [4]. For example, the rate of resistance to ciprofloxacin, an antibiotic commonly used to treat urinary tract infections, varied from 8.4% to 92.9% for Escherichia coli and from 4.1% to 79.4% for Klebsiella pneumoniae in countries reporting to GLASS.
The greatest challenge we face is one of human health. Infections that within our lifetimes have been so easy to treat may soon begin to pose a serious threat. In line with this, a World Health Organization (WHO) report showed how mortality rates are higher in cases of bacterial infections when caused by antibioticresistant strains (Table 1) [5].
| Death (%) | ||||
|---|---|---|---|---|
| Outcome (number of studies included) | Resistant | Not resitant | RR (95% CI) | |
| Escherichia coli resistant to: 3rd gen cephalosporins Fluoroquinolones | Bacterium attributable morality (n=4) Bacterium attributable morality (n=1) | 23.6 0 | 12.6 0 | 2.02 (1.41 to 2.90) |
| Klebsiella pneumoniae resistant to: 3rd gen cephalosporins Carbapenems | Bacterium attributable morality (n=4) Bacterium attributable morality (n=1) | 20 27 | 10.1 13.6 | 193 (1.13 to 3.31) 198 (0.61 to 6.43) |
| Staphylococcus aureus resistant to: Methicilin (MRSA) | Bacterium attributable morality (n=46) | 26.3 | 16.9 | 1.64 (1.43 to 1.87) |
Table 1. Risk of death is higher in patients infected with resistant strains [5]
It is also interesting to note that owing to the differences between healthcare settings and the general community, the antibiotic-resistant strains are also largely specific to each environment (Table 2). That said, although in the past the threat of methicillin-resistant Staphylococcus aureus (MRSA) was once a concern only within the hospital setting, newer strains of MRSA have now been documented as a cause of infections in healthy people in the community.
| Outside hospital | Inside hospital |
• S. pneumoniae (PRSP) | • MRSA, VRSA • Enterococci (VRE) • E. coli (ESBL, NDM-1) • Enterobacter spp. • Glucose-non-fermentative bacteria including Pseudomonas aeruginosa • (MBL, MDRP, Acinetobacter spp.) • Fungi |
Table 2. Examples of drug resistant pathogenic bacteria which cause problems both inside and outside the hospital environment
In addition to impacting on patient outcomes and human health, AMR also presents issues in terms of economic cost. Within the NHS, health budgets are becoming stretched year on year, and with existing treatment options running out, a pragmatic and affordable solution is required for the short term. Within the European Union alone, for example, estimates have suggested that annually, 25,000 deaths and 2.5 million hospital days also converts to an approximate economic burden of €1.5 billion [5]. Globally it has been estimated that failure to address the problem of antibiotic resistance could cost £66 trillion by 2050 (Figure 3) [6].
Figure 3. Health matters: antimicrobial resistance - GOV.UK [6]
The World Bank estimated that if AMR is not addressed, by 2050 the global economy may have lost nearly 4% of annual gross domestic product (GDP), with the losses being even greater in low- and middle-income countries (Figure 4) [7].
As the negative implications of AMR become increasingly clear, a number of approaches have been suggested to overcome the challenges faced.
Figure 4. Substantial and protracted shortfalls in global economic output (Source World Bank (2017). License: Creative Commons Attribution CC BY 3.0 IGO) [7]
The most straightforward solution to this challenge might appear to be the development of novel antibiotics, however, the number of new drugs is small globally (Figure 5) and take so long to reach the clinic that additional methods must also be employed in the short-term. Such methods include preventing the spread of infection within healthcare settings through generic anti-septic means (e.g. copper coatings on surfaces and hand sanitisers). However, key to controlling AMR everywhere is to ensure the most effective use of antibiotics.
Figure 5. Health matters: antimicrobial resistance - GOV.UK [6]
Controlling and encouraging the responsible use of antibiotics is now a major goal of the NHS, as highlighted by its promotion of the Europe-wide public health initiative - European Antibiotic Awareness Day held annually on the 18th November.
The effective use of antibiotics comprises of several different factors:
Every drug compound has its own unique characteristics, including those of its pharmacokinetics (absorption, distribution, metabolism and elimination), which dictates how long the drug stays active in a patient’s system, and pharmacodynamics (its medicinal activity). Both of these properties combined dictate the drug’s antibacterial effect, and it is vital that dosage considers this: while some drugs (e.g. Penicillin) may require a higher number of smaller doses, others (e.g. Azithromycin) instead require a higher total concentration for optimum efficacy [8, 9, 10].
When the patient fails to completely eradicate the whole population of bacteria, those surviving organisms are given a helping hand in evolving protective mechanisms against the drug. When they are allowed to multiply, an entire population of newly resistant bacteria is free to be passed around the community, and it is therefore vital for patients to complete the entire course of antibiotics. Despite it being unfeasible to force patient compliance, it has been shown that compliance is improved during treatments over shorter periods. Treatment can be shortened by increasing the dose, but also by mixing treatments.
As single antibiotic treatments become less effective, another option is to create a cocktail of multiple antibiotic classes for a more effective approach. Such a ‘belt and braces’ tactic hits multiple physiological systems of the bacteria, clearing up the infection more quickly and completely. This approach is of particular interest in paediatrics, to combat the higher resistance rate in young patients, seen partly because the kinds of antibiotics available for small children are limited. As previously noted, shorter treatment times also yield higher patient compliance.
At the present time, antibiotics are generally prescribed freely, even without clinical confirmation of bacterial presence. Unfortunately, differentiating between bacterial and viral infections based on the nonspecific signs and symptoms of many cases can be challenging, for example in respiratory tract infections [11, 12, 13].
Furthermore, unnecessary use of antibiotics can also be attributed to other factors. Physicians need to consider the patient’s anxiety, previous consultation habits, a perception that antibiotics will help, as well as an individual’s need to “legitimise” being ill and off from work or school [14, 15, 16]. Faced with these pressures, physicians often decide to err on the side of caution and prescribe [17].
Taking into consideration the root cause of AMR, it therefore follows that preventing the unnecessary use of antibiotics and restricting the use of higher generation broad-spectrum antibiotics will retain their effectiveness in the long term.
The presence of bacterial infections is indicated by a range of factors, with sepsis, or systemic inflammatory response syndrome (SIRS) traditionally diagnosed based on the occurrence of physiological features such as abnormal body temperature, heart and respiration rate.
As a secondary index, SIRS can also be indicated through blood analysis. While an abnormal white blood cell (WBC) count is a clear indicator, more recently the molecular biomarker C-Reactive Protein (CRP) is being used to enhance blood analysis. The efficacy of CRP and the WBC results have been shown to be medically comparable [18].
While more recent studies have highlighted newer biomarkers of bacterial infections, CRP and the WBC remain cost-effective, with established laboratory standardisation, including the availability of robust internal quality control (IQC) material and widely available external quality assurance (EQA) schemes. These are all highly valuable properties and ideal for the NHS as a whole.
Figure 6. CBC/DIFF + CRP as Biomarkers of infectious care screening
Secreted by the liver in response to a variety of inflammatory cytokines, levels of CRP within the blood therefore provides a significant indicator of inflammation and has become incredibly useful to guide clinical decisions for antibiotic treatments, indicating SIRS if present at a level of >2.0 mg/dl. As shown in Figure 6, a sharp increase in levels is witnessed almost immediately after the onset of inflammation. Measuring CRP levels therefore presents a definite and dynamic indicator throughout the first week of infection.
However, in cases where the CRP level is only marginally raised, differentiating between a viral and bacterial infection can be difficult. Narrowing this grey area, combining CRP with a differential WBC provides more powerful information, in order to guide the decision as to whether the patient needs antibiotics. With CRP’s short half-life, its measurement on an ongoing basis can also be used for monitoring the efficacy of each treatment regime.
Generating this information, in order to decide which cases warrant antibiotic treatment, in addition to selecting the most effective drug, however, requires appropriate diagnosis and evaluation techniques. Importantly, diagnostic methods must be appropriate for point-of-care testing (POCT). For example, a potential solution within the UK involves bacterial testing during Primary Care prior to antibiotic prescribing, using rapid point-of-care assays that quickly guide decision making for each patient. Such technologies are becoming increasingly popular.
Until relatively recently, patient samples had to be sent to the laboratory for testing, but with the surprisingly fast progression of bacterial infections, a quick response offered by new point-of-care assays is highly beneficial, transforming the way blood analysis is performed.
In fact, the UK’s current Longitude Prize which aims to drive new technologies in areas of high significance selected this very topic in 2014, highlighting just how vital this approach is to healthcare. This challenge to help solve the problem of global antibiotic resistance is being run by Nesta and supported by Innovate UK as a funding partner [see Information Box 1].
A vote from the UK public: Antibiotic focus for the Longitude Prize History: In 1714 the British government threw down the gauntlet to solve the greatest scientific challenge of the century – how to pinpoint a ship’s location at sea by knowing its longitude. Today’s challenge: 300 years on and the British public chose Antibiotics to be the focus of the ongoing Longitude Prize introduced in 2014. The challenge is to create a cost-effective, accurate, rapid, and easyto-use point-of-care test for bacterial infections that will allow health professionals worldwide to administer the right antibiotics at the right time. So, conserving antibiotics for future generations. More information can be found online at https://longitudeprize.org |
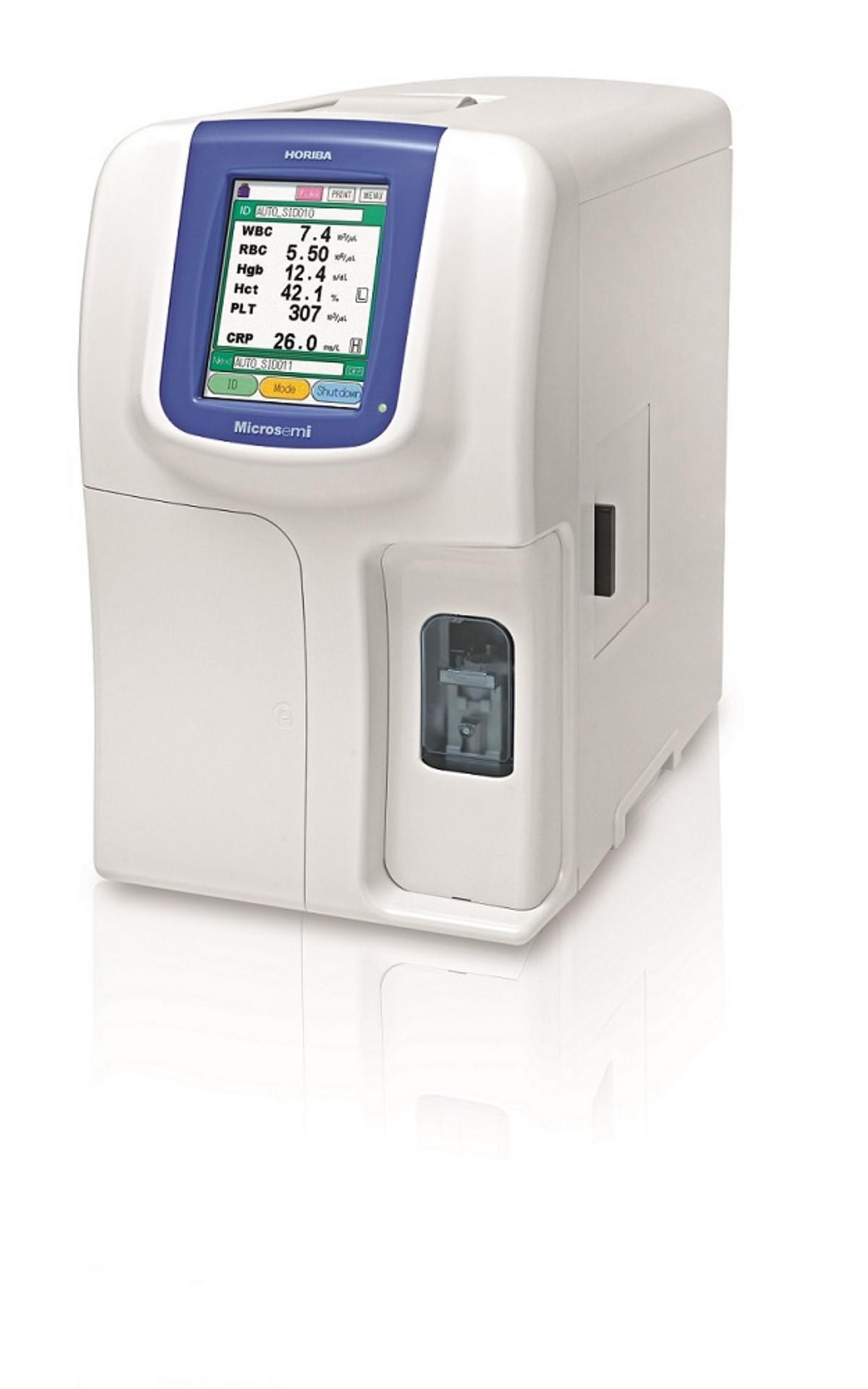
Figure 7: The Microsemi CRP analyser (HORIBA Medical) enabling simultaneous measurement of CRP with complete blood count at the point-of-care
New technologies have already enabled POCT with rapid result generation, meaning that tests can be carried out at the point when Triage or Treatment decisions are made. This approach is especially valuable in the context of differentiating between viral and bacterial infections, and therefore antibiotic prescriptions, and this is where CRP and blood cell analysis comes into play. Immediate availability of CRP results using POC devices, such as the Microsemi CRP analyser (HORIBA Medical), have been shown to reduce antibiotic use [19].
The Microsemi CRP (Figure 7) has been evaluated and confirmed to deliver rapid differentiation between viral and bacterial infection, simultaneously providing a complete blood count (CBC) from 10 μL and CBC+CRP from 18 μL of blood within four minutes [20].
While precise cellular identification is achieved through the electronic impedance variation method, haemoglobin is measured with photometry and CRP with immunoturbidimetry technology. Immediate CRP analysis allows the screening of patient samples to detect the presence of inflammation caused by bacterial infections, drastically reducing the unnecessary use of antibiotics.
The system also incorporates a number of additional features, important when integrating this into primary care units for first-line medical treatments. For example, it can be easily operated by anyone, not just clinical pathology scientists. It is also compact and lightweight, taking up minimum space in busy departments. Its strength lies in its accessibility for widespread applications, and a particular area where antibiotic resistance is a major priority is in the treatment of infants.
One excellent example of the benefits of the application of CRP testing is demonstrated in the paediatric environment. With their immune system not yet fully developed, new-borns are especially susceptible to bacterial infections. Since CRP does not cross the placenta, it presents an unbiased parameter to indicate infection in new-borns, with no interference from maternal blood.
New-borns can also be extremely fragile and taking the smallest blood samples in the least invasive way possible is highly beneficial in the clinical setting. Blood samples for HORIBA Medical’s Microsemi CRP can be taken from a capillary (heel or ear) and requires no specialist nurses to draw no more than 18 μL of blood.
Consequently, this easy-to-use POCT CRP analyser is now being used widely in paediatric settings with notable cost and time savings enabled through optimised discharge time, prevention of unnecessary admissions and helping to control antibiotic administration. A number of recent studies in multiple UK emergency paediatric units, including the Chelsea and Westminster Hospital (London), John Radcliffe Hospital (Oxford), Stoke Mandeville Hospital (Aylesbury) and Wexham Park Hospital (Slough) have demonstrated these benefits [21, 22].
With the latest data and intense focus from organisations such as the NHS and the UK Government promoted Longitude challenge, it has become clear that in order to reduce AMR, strategies for limiting antibiotic use are vital and needed right now.
Through implementing a range of approaches and focusing on POC testing, this goal is entirely achievable, as highlighted by a recent review on the use of POCT for measuring CRP to reduce diagnostic uncertainty and enhance antibiotic stewardship [23]. Testing for CRP using POCT devices such as the HORIBA Medical Microsemi CRP device, delivers rapid, accurate and convenient results, so supporting ‘bedside’ diagnosis of patients in order to determine whether antibiotics are necessary.
As the World Organisation states, “No action today, no cure tomorrow”, and only through changing our approach to antibiotic prescribing in the present will we maintain effective treatment strategies in the future.
References
1 IACG. (2019). No time to wait: Securing the future from drug-resistant infections (www.woah.org//static.horiba.com/fileadmin/Home/eng/Media_Center/docs/pdf/IACG2019/IACG_final_report_EN.pdf)
2 Public Health England (2020). English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR). Report 2019 to 2020 (https://webarchive.nationalarchives.gov.uk/ukgwa/20211022024510/https:/www.gov.uk/government/publications/english-surveillance-programme-antimicrobial-utilisation-and-resistance-espaur-report)
3 UK Health Security Agency (2021). English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR). Report 2020 to 2021 (https://assets.publishing.service.gov.uk/government//static.horiba.com/uploads/system//static.horiba.com/uploads/attachment_data/file/1118310/ESPAUR-report-2021-to-2022.pdf)
4 World Health Organisation (2021). Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2021(https://www.who.int/publications/i/item/9789240027336)
5 World Health Organisation, (2014). Antimicrobial Resistance Global Report on Surveillance 2014 (https://www.who.int/publications/i/item/9789241564748)
6 UK Government (2015). Health matters: antimicrobial resistance (https://www.gov.uk/government/publications/health-matters-antimicrobial-resistance/health-matters-antimicrobial-resistance)
7 Jonas OM, et al. (2017). Drug-resistant infections: a threat to our economic future (Vol. 2): final report (English). HNP/Agriculture Global Antimicrobial Resistance Initiative Washington, D.C.: World Bank Group. (http://documents.worldbank.org/curated/en/323311493396993758/finalreport)
8 Drusano GL & Craig WA (1997). Relevance of pharmacokinetics and pharmacodynamics in the selection of antibiotics for respiratory tract infections. J Chemother.;9:38-44
9 Drusano GL et al., (1998). Pharmacokinetics and pharmacodynamics of fluoroquinolones. Clin Microbiol Infect.;4:27-41
10 Vogelman B et al. (1988). Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J Infect Dis;158:831-847
11 De Sutter A et al., (2006). Predicting prognosis and effect of antibiotic treatment in rhinosinusitis. Ann Fam Med.;4(6):486–493
12 Hopstaken RM, et al. (2005). Clinical items not helpful in differentiating viral from bacterial lower respiratory tract infections in general practice. J Clin Epidemiol;58(2):175–183
13 Macfarlane J, et al., (2001). Prospective study of the incidence, aetiology and outcome of adult lower respiratory tract illness in the community. Thorax;56(2):109–114
14 Cunney R (2001). Strategy for the Control of Antimicrobial Resistance in Ireland (SARI). Dublin, Ireland: National Disease Surveillance Centre
15 Macfarlane J, et al. (1997). Prospective case-control study of the role of infection in patients who re-consult after initial antibiotic treatment for lower respiratory infection in primary case. BMJ.;315:1211–1214
16 Holmes WF, et al., (1997). The influence of antibiotics and other factors on re-consultation for acute respiratory tract illness in primary care. Br J Gen Pract.;47(425):815–816
17 Little P, et al. (2004). Importance of patient pressure and perceived pressure and perceived medical need for investigations, referral, and prescribing in primary care: nested observational study. BMJ;328(7437):444
18 Caldas JP, et al. (2008). Accuracy of white blood cell count, C-reactive protein, interleukin-6 and tumor necrosis factor alpha for diagnosing late neonatal sepsis. J Pediatr (Rio J);84(6):536 42
19 Takemura Y, et al. (2004). Immediate Availability of C-Reactive Protein and Leukocyte Count Data Influenced Physicians’ Decisions to Prescribe Antimicrobial Drugs for New Outpatients with Acute Infections. Clinical Chemistry; 50(1):241-244
20 Woolley, T (2014). A Comparison Between the Horiba Microsemi Point-of-Care C-Reactive Protein and Full Blood Cell Analyzer and the Horiba Pentra 120 and Roche Cobas 6000. Point of Care;13:66–69
21 Yorke H & Cooper H (2016). Improving patient management and healthcare in a paediatric emergency department using combined FBC and CRP point of care testing. Int. Jnl. Lab. Hem. 2016 38 (Suppl. 2): 118
22 McDonald C, et al. (2017). Can using point of care blood tests in emergency paediatric units improve quality of care? Oxford Academic Health Science Network
23 Martinez-Gonzalez NA, (2020). Point-of-Care C-Reactive Protein Testing to Reduce Antibiotic Prescribing for Respiratory Tract Infections in Primary Care: Systematic Review and Meta-Analysis of Randomised Controlled Trials. Antibiotics;9, 610
Do you have any questions or requests? Use this form to contact our specialists.
![Figure 1. Source (ESPAUR report 2019-2020) [2] Figure 1. Source (ESPAUR report 2019-2020) [2]](http://static.horiba.com/fileadmin/Horiba/_processed_/8/9/csm_Figure_1._Source__ESPAUR_report_2019-2020__531917f750.jpg)
![Figure 2. Source (ESPAUR report 2019-2020) [2] Figure 2. Source (ESPAUR report 2019-2020) [2]](http://static.horiba.com/fileadmin/Horiba/_processed_/f/a/csm_Figure_2._Source__ESPAUR_report_2019-2020__d86b762c68.jpg)
![Figure 3. Health matters: antimicrobial resistance - GOV.UK [6] Figure 3. Health matters: antimicrobial resistance - GOV.UK [6]](http://static.horiba.com/fileadmin/Horiba/_processed_/f/7/csm_Figure_3._Health_matters_-_antimicrobial_resistance_-_GOV.UK_b38f19aad3.jpg)
![Figure 4. Substantial and protracted shortfalls in global economic output (Source World Bank (2017). License: Creative Commons Attribution CC BY 3.0 IGO) [7] Figure 4. Substantial and protracted shortfalls in global economic output (Source World Bank (2017). License: Creative Commons Attribution CC BY 3.0 IGO) [7]](http://static.horiba.com/fileadmin/Horiba/_processed_/0/e/csm_Figure_4._Substantial_and_protracted_shortfalls_in_global_economic_output_94a2e615ff.jpg)
![Figure 5. Health matters: antimicrobial resistance - GOV.UK [6] Figure 5. Health matters: antimicrobial resistance - GOV.UK [6]](http://static.horiba.com/fileadmin/Horiba/_processed_/5/8/csm_Figure_5._Health_matters_-_antimicrobial_resistance_-_GOV.UK_434b437ad4.jpg)