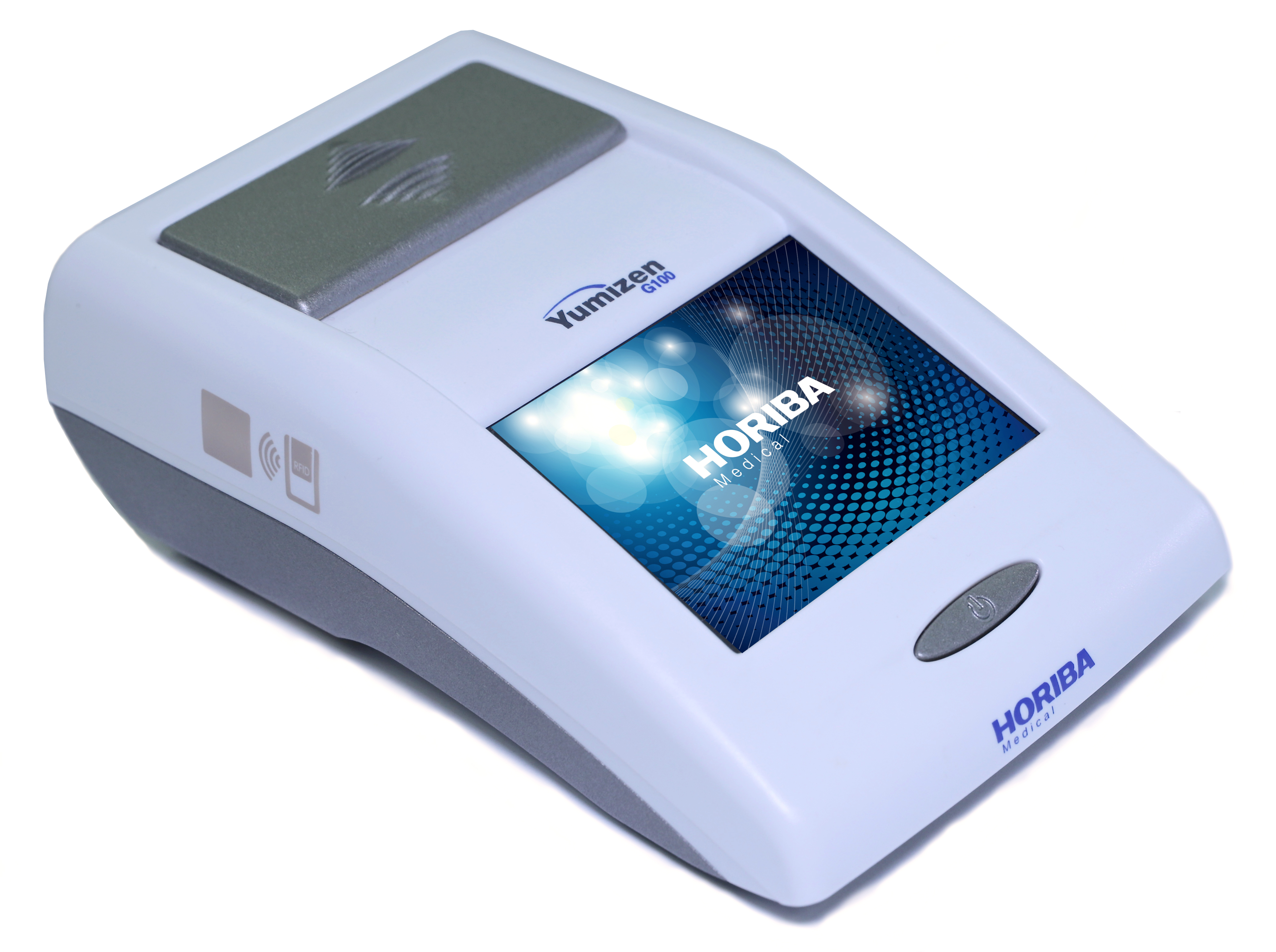
| Dimensions & Weight | Lenght: 102 mm, Height: 55 mm, Depth: 165 mm Weight: 0.38 Kg |
| Printer | Optional with Yumizen G data software |
| Throughput | 120 sec maximum measurement Sampling volume: 20µL |
| Temperature | 15°C - 32°C |
| Power | 100-240v 50Hz-60Hz 6 x 1.5 AA batteries |
| Screen | Color LCD touch screen 53 x 71 mm |
| Storage | 1\'000 results storage capacity |
| Parameter | INR |
| Consumables (kit) | Sampling pipette, 20µl (pink) Dispensing pipette, 200µl (teal blue) Pipette holder 25 capillary samplers 96 pipette tips |
| Reagents | 25 dedicated measurement cuvettes (mixing bead included) Reagent 2 (6 ml) Control l vial (contains lyophilized level 1 plasma control to be dissolved in 200 µl injection grade water) Control ll vial (contains lyophilized level 1 plasma control to be dissolved in 200 µl injection grade water) |
| Linearity | INR: 0,8 - 8,0 |
| Norms | EN 61326-2-6: 2006 IEC 61010-1: 2001 IEC 61010-2-101: 2002 EN ISO 13485: 2003 and 2012 NF EN ISO 14971: 2013 |
| Directives | EN ISO 13485: 2003 98/79/EC (DIV) 2011/65/EU (RoHS) 2002/96/EC (WEEE) et 2006/66/EC (Batteries & Accumulators) 1907/2006 (REACH) 2004/108/CE (Electro Magnetic Compatibility) 2006/95/CE (Voltage limits) |
 해당 제품은 단종되었습니다. 이 페이지는 정보서비스 목적으로만 이용할 수 있습니다.
해당 제품은 단종되었습니다. 이 페이지는 정보서비스 목적으로만 이용할 수 있습니다.
Yumizen G100
Hemostasis Analyzer
Whole Blood Point of Care System
The smallest analyzer for point of care laboratories to monitor oral anticoagulant.
Smart Way to Test
• Dedicated safety lancet
• Single use reaction cuvette
• Innovative RFID reagent identification
• QC Management
Reduce Cost of Care
• Disposable single-test reagent
• Whole blood analysis
• Only one drop capillary blood needed
Increase Efficiency
• INR Screening test based on reference measurement method
• Designed for professional users
• 120 sec measurement
User Friendly Features
• 1'000 data storage capacity
• Mobile device (internal batteries)
• Multiple connectivities
Laboratory information system
Optional Yumizen G data software
*This product may not be available in your country or region at this time. Please contact your HORIBA Medical sales representative or distributor for more information.