Meaning of pH

Let us describe how JIS (Japan Industrial Standard) standards deine pH.
Excerpt from JIS Z 8802-2006
Meaning of pH
In this standard, pH means a value determined based on the definition of the pH scale. It does not have any strict physical and chemical meaning.
Notes: A particular solution, such as a buffer solution with less than 0.1 mol/l with pH ranging from 3 to 10 is assumed to be as shown in formula(1)
Definition of the pH Scale
When representing the pH values of two solutions, with solution X and solution S at the same temperature, for pH (X) and pH (S), the difference between those pH values is defined by formula (2)
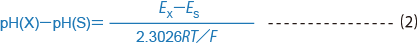
where Ex is the electromotive force of a battery with a glass electrode and reference electrode placed in solution X, and Es is the electromotive force of a battery with a glass electrode and reference electrode placed in solution S.
R: gas constant of 8.3144J/.C¥ mol
T: absolute temperature of t.C+ 273.15
F: Faraday constant of 96485 C/mol
In formula (2), the same units of measure must be used in the denominator and numerator.Table 1 shows values for 2.3026 RT/F in mV at each temperature.
Table 1--Values of 2.3026RT/F
| Temp(.C) | 2.3026RT/F mV | Temp(.C) | 2.3026RT/F mV |
|---|---|---|---|
| 0 | 54.19 | 50 | 64.11 |
| 5 | 55.19 | 55 | 65.11 |
| 10 | 56.18 | 60 | 66.1 |
| 15 | 57.17 | 65 | 67.09 |
| 20 | 58.16 | 70 | 68.08 |
| 25 | 59.15 | 75 | 69.07 |
| 30 | 60.15 | 80 | 70.07 |
| 35 | 61.14 | 85 | 71.06 |
| 40 | 62.13 | 90 | 72.05 |
| 45 | 63.12 | 95 | 73.04 |
The definition represented by formula (2) means that the pH of all solutions are measured in reference to the known pH of a reference solution. That solution is 0.05 mol/l phthalate solution. the pH of which is 4.000 at 15℃
JIS and other standards and regulations relating to pH, such as JIS Z8802, will also be described on the pages dealing with Regulations and pH meters.
Next page Measuring pH Using a Glass Electrode
