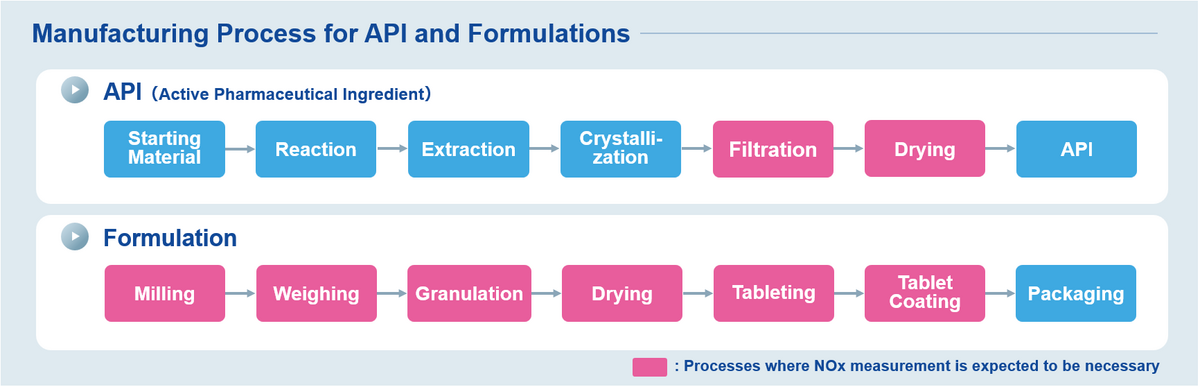
HORIBA's highly accurate trace gas analyzers can continuously monitor N compounds (NOx, NO2, NO) to help clarify the cause of nitrosamine formation during the process of API* and formulation of pharmaceutical manufacturing.
Recently, nitrosamines, which are likely carcinogenic to humans, have been detected globally in the pharmaceuticals such as Sartan class of drugs. The European Medicines Agency (EMA) and the United States Food and Drug Administration (FDA) recommend efforts to prevent the formulation of nitrosamines. Additionally, Japanese Ministry of Health, Labour and Welfare (MHLW) has notified pharmaceutical manufacturers and distributors to conduct self-inspections to assess the risk of nitrosamine contamination. The mechanism of nitrosamine formation is still under research, but one hypothesis suggests atmospheric N compounds could cause nitrosamine formation when ambient air is heated and used for drying formulation during the granulation process.
*API: Active Pharmaceutical Ingredient
Ưu điểm của APNA-380
Máy kiểm tra oxit nitơ APNA-380
Máy kiểm tra oxit nitơ
Máy kiểm tra Amoniac trong môi trường xung quanh
Máy phân tích phân bố kích thước hạt tán xạ laser
Bạn có thắc mắc hoặc yêu cầu nào không? Hãy sử dụng mẫu này để liên hệ với các chuyên gia của chúng tôi.