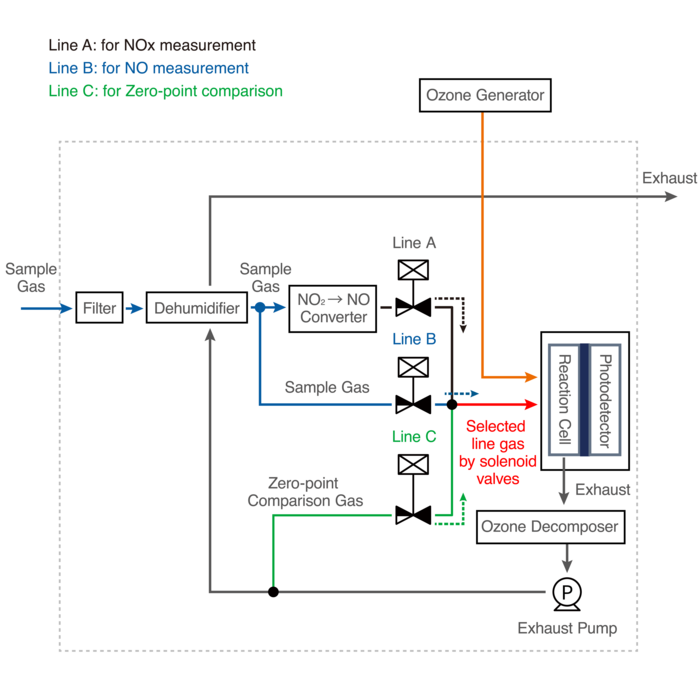
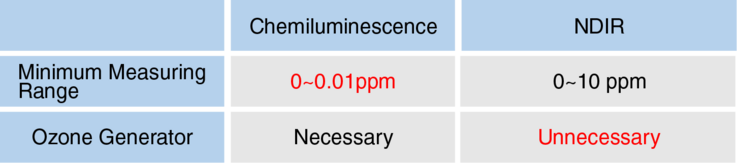
Measuring Principles
What is the Chemiluminescence Method ?
Chemiluminescence is a phenomenon in which molecules excited by a chemical reaction emit excitation energy as light when they return to their ground state.
For example, when nitrogen monoxide (NO) reacts with ozone (O3) and is oxidized, NO changes to nitrogen dioxide (NO2). The resulting NO2 promotes to an excited state (NO2) at a fixed rate and emits at a specific wavelength when it returns to the ground state of NO2. (Equation 1)
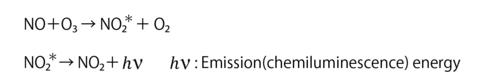
Equation 1: Chemiluminescence with a Chemical Reaction
This principle is used for continuous concentration measurement of nitrogen oxides (NOx: NO + NO2), NO, NO2 and ammonia (NH3) in sample gases.