Before antimicrobials were a widespread phenomenon, a simple scratch could be fatal, if infected. Nowadays, we are heavily protected from infection due to better hygiene practices, vaccines, and anti-microbials. However, the antimicrobials that we have relied upon for the last 100 years are becoming less effective. This is due to antimicrobial resistance (AMR).
The World Health Organisation (WHO) has highlighted that drug-resistant bacterial infections contributed to an estimated 4.95 million associated deaths in 2019, including 1.27 million deaths directly attributable to bacterial AMR, making AMR one of the top 10 public health threats globally [1, 2]. Also, the UK government has estimated that if action is not taken, by 2050 AMR could result in at least 10 million extra deaths a year globally at an economic cost of £66 trillion [3].
Up to 2020, WAAW was known as World Antibiotics Awareness Week, however, to underline a broader scope which encompasses all antimicrobials, including antibiotics, antifungals, antiparasitics and antivirals, WHO has renamed it World AMR Awareness Week. This also reflects that AMR is a complex issue requiring a united multi-disciplinary approach known as the One Health approach. AMR is not only a threat to humans, but also animal, plant and environmental health and is the reason that WAAW 2023’s theme is “Preventing Antimicrobial Resistance Together” using the slogan “Antimicrobials: Handle with Care.”
The Evolution of AMR
Infectious diseases were the leading cause of death worldwide before 1900, particularly pneumonia, influenza, and tuberculosis. However, the successful implementation of public health initiatives, vaccine introductions, and the development of antimicrobial compounds, starting with penicillin in 1928, greatly reduced the mortality rate from infectious diseases.
But, after decades of overuse and misuse of antimicrobials, an increasing number of infections caused by bacteria, parasites, viruses and fungi are becoming drug-resistant. This is due to microorganisms responding to their environment just like any organism, mutating and evolving to protect themselves against the toxic compounds that they are exposed to.
Preventing AMR spread together
In line with WHO, the UK government recognises the need for urgent action and has committed to containing and controlling AMR by 2040 [4]. This plan also acknowledges the need for a global and national multi-sectoral effort to lower the burden of infection, optimise the use of antimicrobials, improve surveillance data, and to develop new diagnostics and treatments.
As antimicrobial resistant microbes can spread between human, animal and plant populations, and through their shared environment too, so the aforementioned One Health approach is needed. This brings together many disciplines in a joint effort to provide solutions for human, animal, and environmental health (Figure 1).

Figure 1. The One Health approach
AMR is linked to all these components due to the prolific use of antimicrobials in areas including human healthcare, livestock production, agriculture, and aquaculture. Antimicrobials are used in these industries to prevent disease and maintain animal welfare and food security. However, this perpetuates the occurrence and spread of AMR, threatening food supply, human health, and veterinarian treatments.
AMR is a complex and global concern encompassing issues such as antimicrobial mismanagement, inadequate infection control, agricultural debris, contaminants in wastewater, and the movement of people and animals infected with resistant microbes. So, the management of antimicrobials must be reduced and regulated across sectors including: healthcare facilities; farms and food industry premises; sewage treatment plants; and in the management of waste from antibiotic production.
Consequently, within the UK, the UKHSA is leading the One Health Antibiotic Guardian campaign working with partner organisations, such as the Veterinary Medicines Directorate and Food Standards Agency, providing AMR information and resources for use across all sectors. In turn, other organisations within these sectors are developing their own campaigns. For example, the PROTECT ME guidelines supporting the responsible use of antibiotics in veterinary practice are being actively promoted by the BSAVA and BEVA [5, 6].
POCT supporting appropriate prescribing
The excessive and improper use of antibiotics in people and animals is seen as a major driver of AMR. This is because antibiotics are often prescribed without clinical confirmation of bacterial presence, so, many infections are treated with antibiotics inappropriately if they are viral.
Diagnostic tests are the primary means of identifying infectious disease in humans and animals and detecting resistant microorganisms. However, traditional microbial detection methods generally involve sending samples for laboratory testing, which is time-consuming and delays diagnosis, this can lead to further disease spread, or use of inappropriate medication.
To close the gap between treatment and diagnosis, NHS England has recently published new guidance on integrating point-of-care testing (POCT) diagnostic technologies into urgent community response and virtual ward services, to ensure the best clinical outcomes [7]. This guidance emphasises the importance of effective, fast, and low-cost diagnostic technologies for guiding the appropriate use of antibiotics. Use of POCT is proving to be highly beneficial, transforming the way that diagnostics are performed and delivering immediate results to enable informed clinical decision making for patients prior to antibiotic prescribing.
POC diagnostics in action
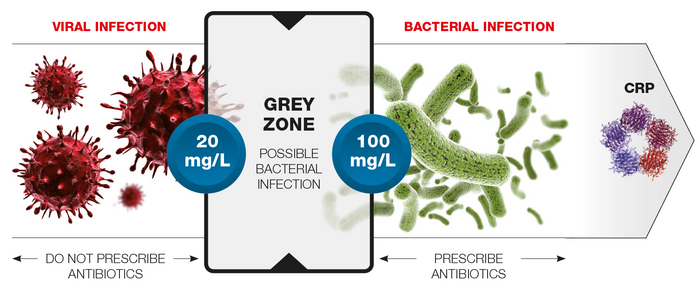
To differentiate between bacterial and viral infection, the inflammatory biomarker, C-reactive protein is widely measured in conjunction with a Full Blood Count (FBC). Traditionally, these tests are performed in the hospital laboratory and, once received in the lab, results can take up to 60-90 minutes. The actual time from needle to result delivery can be much longer though. In situations where patients are particularly susceptible to bacterial infections, such as newborns and children, POCT can be invaluable to support fast prescribing decisions.
In a recent Oxford Academic Health Science Network study, the John Radcliffe (JRH), Stoke Mandeville (SMH) and Wexham Park (WPH) hospitals investigated whether the introduction of POC testing for simultaneous CRP and FBC, using HORIBA’s Microsemi CRP haematology analyser, could provide more rapid decision-making in a range of common paediatric conditions [8]. The study found that using POCT as a replacement for laboratory tests resulted in earlier decision-making in approximately 75% of cases across three hospital sites. Economic analysis across these sites found that this could result in net annual cost savings due to reduced staff time, as well as faster decisions on antibiotic use.
HORIBA’s POCKIT Central veterinary PCR analyser also offers an example of how POCT can support fast in-house veterinary diagnostics and biosecurity without sending samples to external laboratories. It uses fully automated PCR testing to rapidly indicate the presence of viral, bacterial, protozoal and parasitic infections for up to eight pathogens in one run. With nearly 200 assays available across small animal, equine, farm and exotic practice, POCKIT can inform within 85 minutes the correct treatment and management choices, so supporting AMR reduction.
Looking to the future
The widespread use of antimicrobials for decades has spawned a new generation of antimicrobial-resistant strains (aka ‘superbugs’). Consequently, treatment options for common infections are running out and AMR now presents a major health issue globally, with a high proportion of resistance reported within both healthcare settings and the general community. This means routine surgery and many immune-suppressing cancer treatments could soon become simply too dangerous to perform due to infection risk.
By implementing a range of One Health approaches and use of POCT it is possible to limit inappropriate antibiotic use and help to reduce AMR. Incorporating POCT into routine practice, healthcare providers can identify the causative agent of an infection quickly and accurately, enabling evidence-based decisions for antibiotic prescriptions. This not only helps reduce the development of resistance, but also ensures patients are receiving the best care.
WHO has stated, “No action today, no cure tomorrow”, we can’t take antimicrobials for granted. Only through changing our approach to antibiotic prescribing in the present will we maintain effective treatment strategies in the future. You can find out how you can take action today on WHO’s WAAW 2023 page: www.who.int/campaigns/world-amr-awareness-week/2023
Read more about AMR and how evidence-based diagnosis can support its reduction in HORIBA’s ‘Man versus Microbes’ white paper
References:
- Murray, C. J. L., et al. (2022). Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. The Lancet, 399(10325), 629–655. doi.org/10.1016/S0140-6736(21)02724-0
- World Health Organization (2020). 10 global health issues to track in 2021. www.who.int/news-room/spotlight/10-global-health-issues-to-track-in-2021
- O’Neill, J. (2016). Tackling drug-resistant infections globally: Final report and recommendations (United Kingdom) [Report]. Government of the United Kingdom. apo.org.au/node/63983
- HM Government (2019). Contained and controlled. The UK’s 20 year vision for antimicrobial resistance. www.gov.uk/government/publications/uk-20-year-vision-for-antimicrobial-resistance
- British Small Animal Veterinary Association (BSAVA). PROTECT ME. Accessed from www.bsava.com/resources/veterinary-resources/protect-me/ (November 2023).
- British Equine Veterinary Association (BEVA). Protect Me Toolkit. Accessed from www.beva.org.uk/Protect-Me (November 2023).
- NHS England (2023). Integrating in vitro point of care diagnostics: Guidance for urgent community response and virtual ward services (2023, August 29). www.england.nhs.uk/long-read/integrating-in-vitro-point-of-care-diagnostics-guidance-for-urgent-community-response-and-virtual-ward-services/
- Hart, J. (2021). Opportunities for point of care testing. Oxford Academic Health Science Network. www.oxfordahsn.org/wp-content/uploads/2021/10/AHSN-point-of-care-final.pdf