

The quality of brewing water affects the enzyme activity in mash, solubility of minerals, taste and quality of beer, and the condition of brewing equipment. To achieve the great taste of beer and ascertain the cleanliness of equipment after cleaning process, water quality check must be carried out in the various stages of brewing process with reliable and accurate instruments. Having the advantages of less maintenance design, low-volume sample requirement, and hassle-free operation, the HORIBA LAQUAtwin pocket meters and LAQUA dissolved oxygen (DO) handheld meters and electrodes are recommended for home and commercial beer brewers.

Beer is one of the most widely consumed alcoholic drinks and the third most popular drink overall (after water and tea) in the world. It has become a part of the culture of many nations and often associated with social traditions such as festivals and games.1
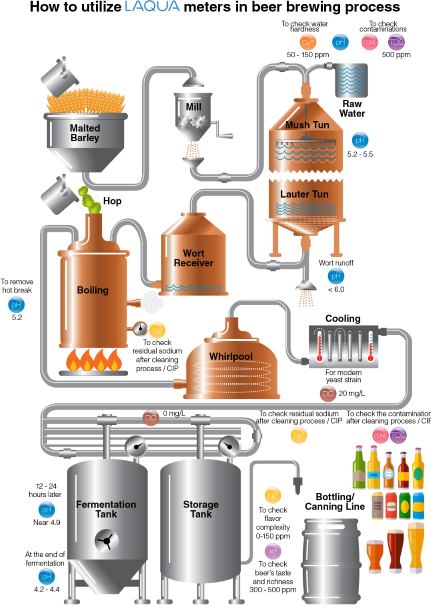
Brewing is the process of making beer. The four main ingredients of beer are cereal grains (e.g., malted barley, wheat, maize, rice) as starch source, water, flavoring agents (e.g., hops, gruit, herbs, fruits), and yeast. Malted barley and hops are the most commonly used cereal grain and flavoring agent, respectively. In beer brewing, the starch source is mixed with water and converted into a sugary liquid called wort. The wort is subsequently converted into the alcoholic drink by fermentation using yeast.
As beer is mostly water, the composition of brewing water can affect the quality of beer. Brewing water should be clean and odorless. Water can come from two sources—surface water and groundwater. The former is low in dissolved minerals but higher in organic matter while the latter is generally higher in dissolved minerals and low in organic matter.2 To determine the mineral composition of water, beer brewers can ask reports from local water companies, send sample water to laboratories, or perform the tests by themselves using proper equipment. The common test parameters are explained below. Based on the results, brewers can make necessary water adjustments to make a great beer.
As water quality check in beer brewing process is a crucial step to achieve exceptional taste and quality of beer, the HORIBA LAQUAtwin pocket meters and LAQUA dissolved oxygen (DO) handheld meters and dissolved oxygen (DO) electrodes are recommended for beer brewers. Aside from their simple and easy operation, the LAQUAtwin pocket meters have unique flat sensors that can measure sample as low as 0.05 ml in just few seconds. The LAQUA DO handheld meters are packaged with DO electrodes, which have innovative replaceable DO tips and built-in temperature sensor, in carrying cases for routine measurements in the field.
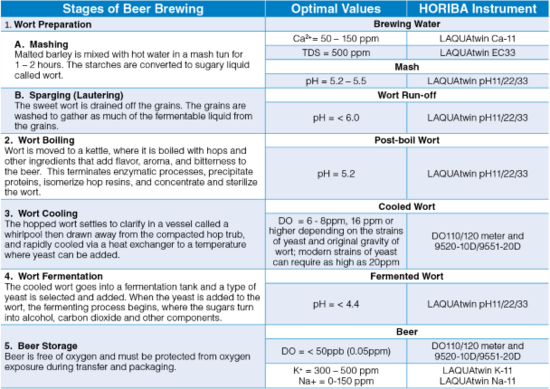
Water hardness is defined as the amount of dissolved calcium and magnesium in the water. Calcium is the principal ion that determines water hardness. It can overcome the buffering capacity of malt phosphates, lower the mash pH to acceptable range3 and promote clarity, flavor, and stability in the finished beer.2 The ideal calcium concentration in the brewing water is between 50 and 150 ppm.3 Calcium sulfate (CaSO4), also known as gypsum, or calcium chloride (CaCl2) can be added to increase the amount of calcium ions in water. Magnesium also contributes to water hardness and therefore affects the mash pH, but to a lesser extent than the calcium.2
Alkalinity is the capacity of water to resist changes in pH that would make the water more acidic. It is a measurement of the concentration of all alkaline substances dissolved in the water such as carbonates and bicarbonates which buffer pH in the water by neutralizing acids.
Calcium concentrations must be balanced with low carbonate-bicarbonate levels as they have countering effect on calcium. These ions should be kept to less than 50ppm. Bicarbonates, being strong alkaline buffers, may raise the pH of the mash to unacceptable levels, if available in large amounts.3
pH is important in various stages of beer brewing—mash, wort, and finished beer. Most municipal water sources are slightly alkaline. When water is combined with grains in a mash tun, the pH of the mixture, called mash, drops compared to the pH of water alone. The mineral composition (i.e., calcium, magnesium, carbonates, and bicarbonate) of the water and natural acidification process in mashing of malted grains drive the mash pH. The ideal mash pH range is 5.2 to 5.5 with preference to the lower end (5.2) for improved enzyme activity leading to optimal conversion of starches to liquid sugars called wort.4 After mashing, the wort is drained and the grains are washed. This process is called sparging. As sparging progresses, the pH of wort being run off from the mash increases. The pH of the run off wort should be below 6 because higher pH will extract tannins, silicates, and other compounds from the grain leading to astringent-off flavors and cloudy, hazy beer.3
The collected wort is transferred to a kettle for boiling. During the boil, calcium phosphate is still precipitated (as long as calcium is still present) and the pH decreases, just like during mashing. A post-boil wort pH of 5.0 to 5.2 should be achieved. This pH range will help extract the best character from hops, maximize the amount of hop break formed, and keep color pick-up to a minimum. Another important function of boiling is coagulating the hot break. The optimal pH for break formation is 5.2. If big, fluffy bits of break material in wort are present in early boiling, it is a confirmation that pH is in the right range.5 Hot break must be removed so that the hot wort can be clear.
When yeast is added to the wort, the fermentation begins—sugar turns into alcohol and carbon dioxide. During fermentation, the pH continues to drop because yeast takes in ammonium ions (strongly basic) and excretes organic acids (includes lactic acid). The yeast strain chosen can affect the final beer pH—most lager beers = pH 4.2 – 4.6, some ales = pH 3.8, sour beers = around pH 3.0. An optimal pH of less than 4.4 favors faster beer maturation, better beer clarity, better biological stability and more refined beer taste.5
Sodium is a desirable ion in beer for final product flavor complexity. The range is 0-150 ppm Na+. Professional brewers use simple cleaning process or clean-in-place (CIP) techniques to make regular cleaning efficient and effective. CIP involves circulating cleanser or sanitizer through a spray ball in enclosed equipment. Brewers will clean each time the wort or beer is transferred from a vessel. For example, kettle must be cleaned after the wort is boiled and sent off through the heat exchanger and into the fermentor. Cleansers with sodium hydroxide (commonly known as caustic soda) as active ingredient are by far the most widely used in breweries.10 Any sodium residue in the equipment after cleaning process / CIP can be checked using an ion selective sensor and meter.
Brewing water from municipal water sources may contain total dissolved solids (TDS) such as minerals, salts, and organic matter. TDS is an indicator of water quality and, as discussed above, mineral composition of water affects mash pH in brewing process. The recommended TDS level in water is 500ppm.6 The amount of TDS in water can be estimated using a TDS meter, which measures the conductivity of solution and converts it to TDS using a factor. Although TDS does not specify the exact mineral composition of water, it can be used in monitoring any changes in the water.
Electrical conductivity (EC), often simply referred to as conductivity, is a measure of the ability of an aqueous solution to carry an electric current. Pure water has no conductivity (reading is not zero but very low) because there are no dissolved ions in it. The more ions in solution, the higher the conductivity value. Conductivity measurement of water source in beer brewing gives an indication of the water purity and the baseline measurement before brewer can make any adjustments, like addition of minerals.
After boiling in the kettle, the hopped wort is devoid of oxygen. It is cooled down rapidly to below 80 °F (27 °C) before oxygenation for better oxygen uptake. The amount of dissolved oxygen required depends on the yeast strain and the original gravity of the wort. Traditional ale and lager worts were usually not collected higher than 1045 (12°P) and required 6 to 8 ppm dissolved oxygen. With high gravity brewing original gravities have increased up to 1080 (20°P) and require dissolved wort oxygen levels of 16 ppm or higher.7 Modern strains of yeast can require as high as 20ppm DO. After oxygenation, yeast is pitched into the wort. Yeast utilizes oxygen to become healthy and to reproduce before fermenting the wort to beer. Oxygen is necessary to produce unsaturated fatty acids and sterols for yeast’s cell walls. A strong cell wall enhances the yeast’ alcohol tolerance. Healthy yeast metabolizes wort into alcohol and carbon dioxide without giving off-flavors or off-odors.8 Once fermentation is complete, the beer is free from oxygen and must be protected from oxygen exposure to prevent oxidation and staling. It is almost impossible not to introduce oxygen during transfers and packaging (bottling and canning), but the amount should be insufficient for healthy yeast to grow.7 Brewers should be capable of achieving less than 50 ppb (0.05ppm) total in package oxygen.
Malt contains between 4-6 g/kg of potassium, the major part of which is solubilized during mashing; in beer the concentration of potassium lies between 300 – 500 ppm (mg/L). Above 500 ppm, beer can be salty. Potassium has a purely saline effect. The potassium/calcium ratio affects yeasts flocculation.9
1. Beer – Wikipedia (Accessed on 27 May 2019 at https://en.wikipedia.org/wiki/Beer
2. Brewing Water (Accessed on 27 May 2019 at beerandbrewing.com/brewing-water/)
3. The Power of pH (Accessed on 27 May 2019 at byo.com/article/the-power-of-ph/)
4. Mash pH and Why It Matters for All Grain Beer Brewing (Accessed on 27 May 2019 at beersmith.com/blog/2015/05/07/mash-ph-and-why-it-matters-for-all-grain-beer-brewing/)
5.The Principles of pH (Accessed on 27 May 2019 at byo.com/article/the-principles-of-ph/)
6. Reading a Water Report (Accessed on 27 May 2019 at howtobrew.com/book/section-3/understanding-the-mash-ph/reading-a-water-report)
7. The Role of Oxygen in Brewing (Accessed on 27 May 2019 at http://www.ibdlearningzone.org.uk/article/show/pdf/494/)
8. Aeration Basics (Accessed on 27 May 2019 at https://www.morebeer.com/articles/Wort_Oxygenation_Aeration)
9. Handbook of Homebrewing by William Hardwick, page 142.
10. The Dirt on Brewery Cleaning (Accessed on 27 May 2019 at www.morebeer.com/articles/Brewery_Cleaning)
11 June 2019, Rev. 0
Pocket Water Quality Meters
Pocket Water Quality Meters
Pocket Water Quality Meters
Pocket Water Quality Meters
Pocket Water Quality Meters
Pocket Water Quality Meters
Pocket Water Quality Meters
Pocket Water Quality Meters
Pocket Water Quality Meters
Sie haben Fragen oder Wünsche? Nutzen Sie dieses Formular, um mit unseren Spezialisten in Kontakt zu treten.
HORIBA Advanced Techno, Co., Ltd.
31, Miyanonishi-cho, Kisshoin
Minami-ku Kyoto 601-8306 Japan
Tel: +(81) 75 321 7184
Fax: +(81) 75 321 7291
HORIBA Instruments (Singapore) Pte Ltd.
83 Science Park Drive, #02-02A,
The Curie 118258 Singapore
Tel: +65 6 908 9660
Fax: +65 6 745 8155
Mail: laqua(at)horiba.com
HORIBA UK Limited Northampton Office
Kyoto Close,Summerhouse Road,
Moulton Park
Northampton NN3 6FL UK
Tel: +(44) 1604 542 600
Fax: +(44) 1604 542 699
Mail: waterquality(at)horiba.com
HORIBA Instruments Incorporated Head Office
9755 Research Drive
Irvine California 92618 USA
Tel: +1 800 446 7422
Fax: +1 949 468 1790
Mail: labinfo(at)horiba.com
You might also like to know...
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
