

Determining the calcium content of drinking water helps consumers accurately gauge their calcium intake. Unlike atomic absorption spectroscopy (AAS) and inductively coupled plasma atomic emission spectroscopy (ICP), the LAQUAtwin calcium ion meter offers a simpler method for measuring calcium ion - ionizing bound calcium in water using acid before analysis.
It is helpful to determine the amount of calcium contained in water, since this will enable one to ascertain whether the water is hard, or if it has minerals. This can be determined using atomic absorption spectroscopy (AA) or inductively coupled plasma atomic emission spectroscopy (ICP). However, a much simpler way is by ionizing acid-bound calcium using acidizing pretreatment. The LAQUAtwin Ca2+ can be used to measure the total amount of calcium.
The LAQUAtwin Ca2+ meter is used as check to determine the calcium content of water products before selling to consumers. This is an easy, quick method used to check the amount of calcium present in water.
As a supporting electrolyte, add 0.0375 g potassium chloride to 5 ml pre-treated sample solution and dissolve by stirring. Measure the sample with LAQUAtwin callcium meter that has been calibrated and use the dilution ratio to calculate the result. Wash the sensor with tap water and pat dry with a paper tissue.
The use of accurate calcium ion testing in controlling the quality and calcium content of water products ensures that consumers are accurately able to gauge their calcium intake. It also enables one to determine whether or not scaling will occur with boiling of their water.
For each type of sample, the following table shows the labeled value, together with the values measured by various methods.
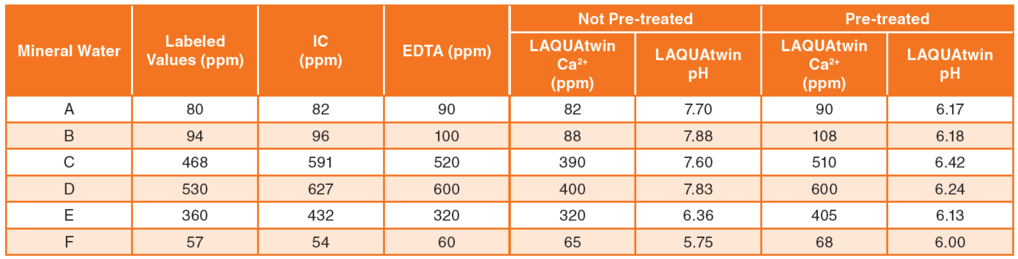
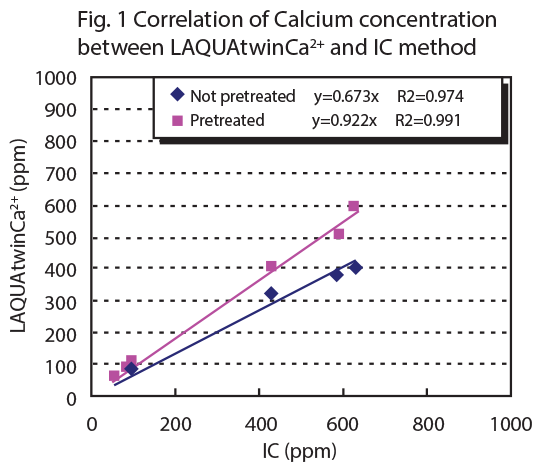
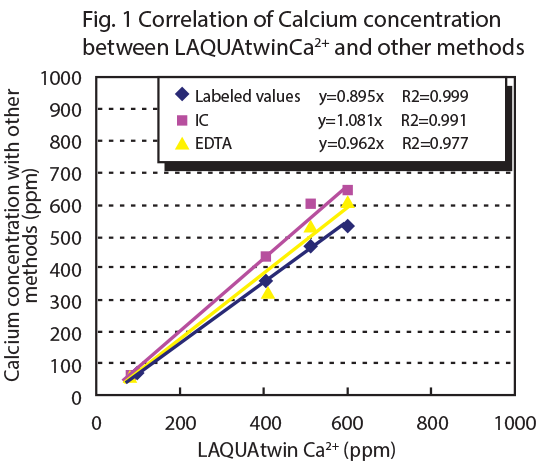
Figure 1 shows the correlation between LAQUAtwin Ca2+ data and that from ion chromotography for pretreated and non pretreated samples. Figure 2 shows the correlation between LAQUAtwin Ca2+ data and that from other methods for a pretreated sample. Calcium rich mineral water contains calcium sulfate and calcium carbonate, and these were ionized by pretreatment. The data for total Ca amount obtained by LAQUAtwin Ca2+ shows better correlation with other methods when the sample has been pretreated.1
1 Internal study by HORIBA labs, 2013
Sie haben Fragen oder Wünsche? Nutzen Sie dieses Formular, um mit unseren Spezialisten in Kontakt zu treten.
Pocket Water Quality Meters

