Hydrogen-Ion Activity
Redefining pH
Sørensen’s definition of pH, i.e. that pH = −log10[H+], was later revised, as further research demonstrated that pH is more related to hydrogen ion activity than hydrogen ion concentration. As a result, pH was redefined as follows in 1920:

This new definition is recognized as the most theoretically correct by the academic and research community. Practical pH measurement also uses methods based on this definition, as explained in the next chapter. Advances in thermodynamics and practical methods of pH measurement have played an important role in this research.
Activity of hydrogen ions
So, what is activity of hydrogen ions? Let's try to clear that up.
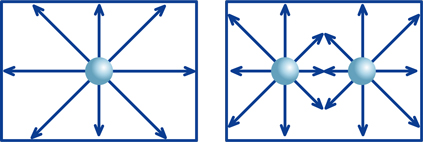
Imagine a box of known size that contains one steel ball. If you move the box back and forth or side to side, the ball rolls around freely within the box. Then, suppose you have two balls in an identical box. These two balls will sometimes collide, which places certain limits on possible directions of movement of the balls. But movement is not severely restricted with only two balls. However, as the number of balls increases, the movement of the balls becomes more and more limited. Suppose we call the degree of this restriction f. If we multiply the degree of restriction f by the number of balls in the box, the result will correspond to the number of balls that have freedom of moment at a given instant.
Next, apply this example to hydrogen ions within a solution, where the balls are hydrogen ions (H+), the number of balls is hydrogen-ion concentration ([H+]) and the number of balls that can move about freely is the activity of hydrogen ions. And "moving about freely" means that an ion can "exert its particular characteristics." We use f as the activity coefficient.
This leads us to the following formula:

Note that the theoretical definition of pH uses the extremely difficult concept of activity, as shown here.

The definition of pH first introduced by Sørensen (the concept that pH is determined by hydrogen concentration) was therefore partly amended as science advanced. However, his definition confers advantages in terms of practical usage, and the corresponding amendment does not downgrade its biological and chemical significance. Advances in thermodynamics and practical methods of pH measurement have played an important role in the process of this redefinition. For this reason, from the point of view of the engineers who use pH, it can still be said that “the Father of pH” is a title that Sørensen deserves. Sørensen’s first definition is still used in basic general chemistry courses, in order to make the concept easier to understand.
Next page Definition of pH