

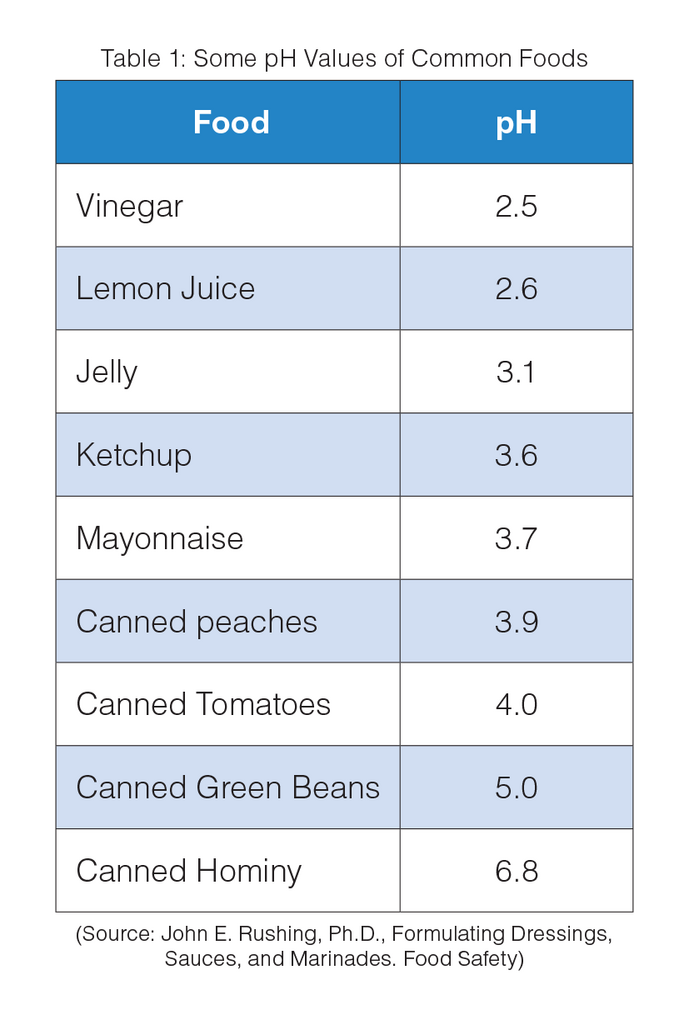
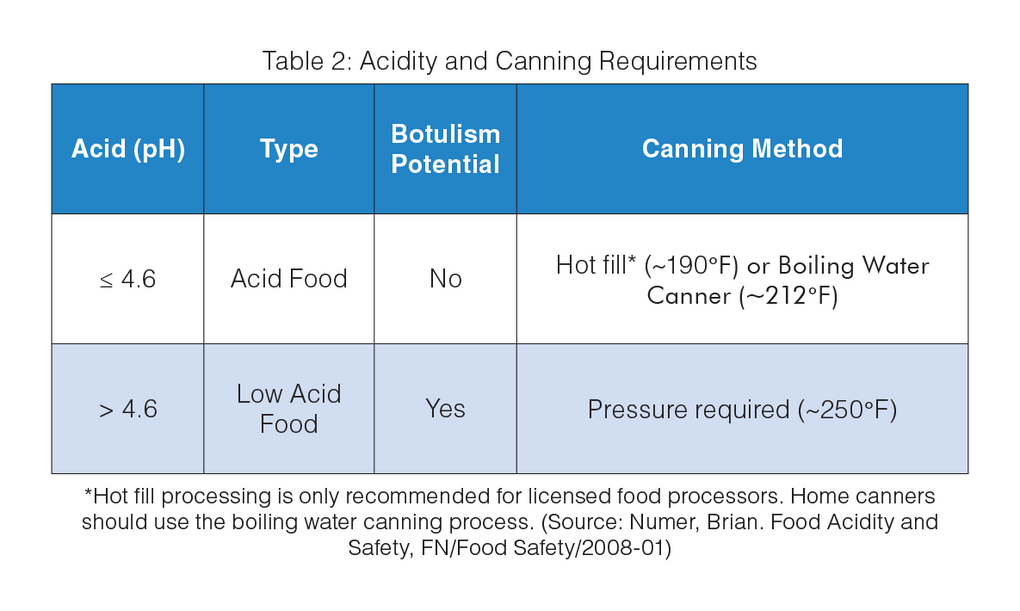
For brine of canned acid foods, the equilibrium pH value must be 4.6 or below to inhibit the growth of Clostridium botulinum, the most heat resistant of the food pathogen microorganisms.
The anaerobic bacterium Clostridium botulinum produced in improperly canned foods has caused illness and death. The vacuum seal on cans provides an oxygen-free environment that will allow C. botulinum spores to grow and produce deadly toxin, if the canning process is not carried out properly. Fortunately, the C. botulinum spores will not grow in high-acid foods (pH ≤ 4.6). For low-acid foods (pH > 4.6), these spores that are resistant to boiling water temperature must be killed during the canning process.
The appropriate pH in the cans is obtained by the use of brines with known acid concentrations or tablets of known acid compositions that are added to cans of specified volumes. The contents of the cans must be then conveniently stirred to ensure that the pH is below 4.6 in the center of all food particles. Acids used in canning to lower the pH to 4.6 are usually citric, lactic and malic, but also glucono-delta-lactone.
The LAQUAtwin pH meter can be used by food processors or home canners to measure the pH of brine in canned foods. There are three (3) LAQUAtwin pH meter models available, namely pH 11, 22, and 33. These light, pocket-sized meters allow two to five calibration points using either NIST or USA pH buffers. The pH 33 meter has built-in temperature sensor that measures and displays temperature and automatic temperature compensation feature (ATC) that performs automatic calibration to the exact pH of the buffer at the measured temperature. Refer to the specifications of each meter model for more information.
Calibrate the LAQUAtwin pH meter using pH 4.01 and 7.00 (or 6.86) buffers according to manufacturer’s instructions.
Sample Measurement
Using the pipette that comes with the meter, take an aliquot of the brine sample and place some drops into the sensor. Record the pH and temperature once stabilized. After testing each brine sample, rinse the sensor with water and blot dry with soft tissue. To obtain accurate results, a uniform temperature should be maintained for the standard buffer solutions and brine samples. The test should be made at a temperature between 20-30°C, the optimum is 25°C.
Food acidity is important in preventing botulism, a foodborne illness that comes from eating contaminated food with toxins produced by C. botulinum. This fact is used in canning acid foods. Aside from following tested recipes and proper canning methods to ensure that C. botulinum is killed and does not grow in canned food, performing accurate pH measurement using a reliable instrument is also necessary to ensure that the correct pH value of 4.6 or below is attained for food safety and regulatory compliance. The final equilibrium pH (the pH of a food product after the food acid is distributed equally throughout the product) must be checked, controlled and documented after the product has completed the thermal processing step.
REV 0, 12 AUGUST 2015
Você tem alguma dúvida ou solicitação? Utilize este formulário para entrar em contato com nossos especialistas.
Pocket Water Quality Meters
Pocket Water Quality Meters
Pocket Water Quality Meters
