

Measuring the salinity or the dissolved salt content of water is important as aquatic organisms, livestock, and crops thrive at different salinity levels. Freshwater has a salinity value of less than 0.5 ppt while seawater has an average salinity of 35 ppt.


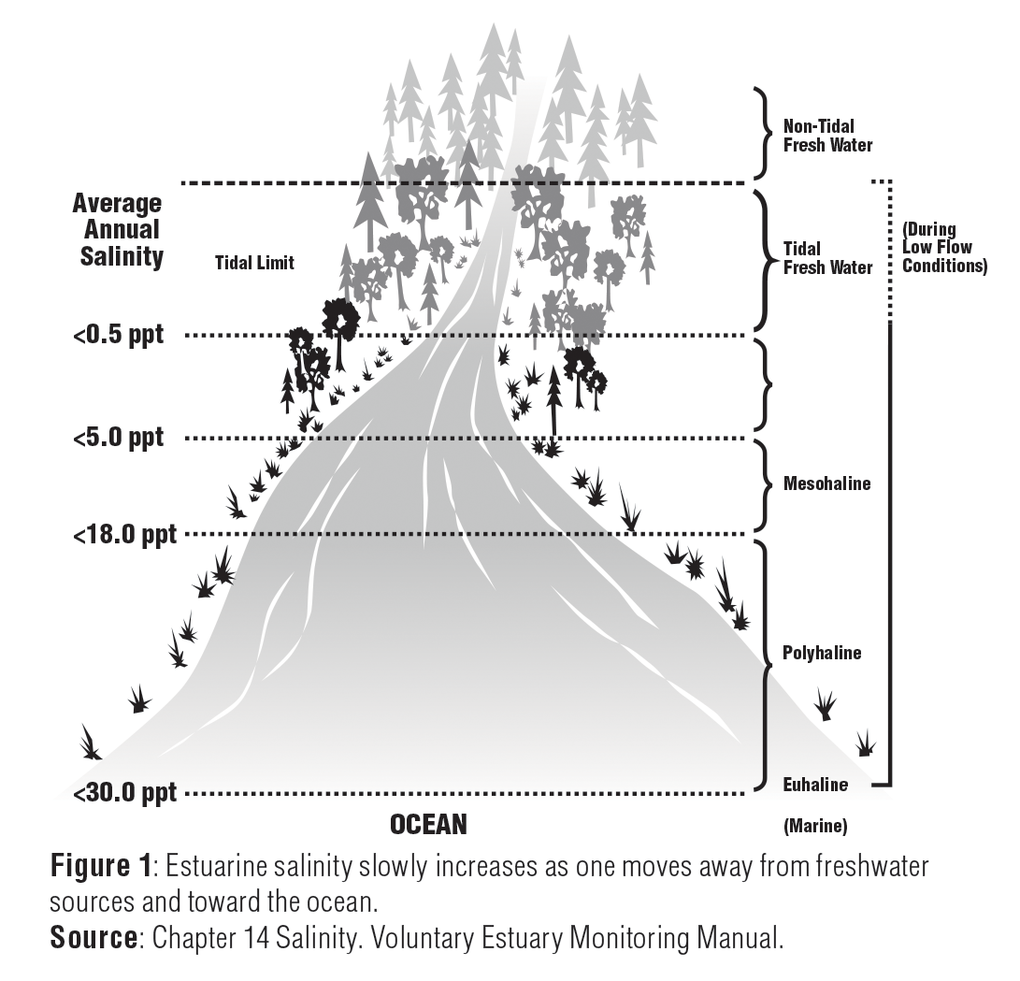
Salinity is the measure of the amount of dissolved salts in water. It is usually expressed in parts per thousand (ppt) or percentage (%). Freshwater from rivers has a salinity value of 0.5ppt or less. Within the estuary, salinity levels are referred to as oligohaline (0.5-5.0 ppt), mesohaline (5.0-18.0 ppt), or polyhaline (18.0-30.0 ppt). Near the connection with the open sea, estuarine waters may be euhaline, where salinity levels are the same as the ocean at more than 30.0 ppt.1
Salinity varies from place to place in the oceans, but the relative proportions of the most major dissolved constituents remain virtually constant. Even though there are smaller quantities of other ions in seawater (e.g., K+, Mg2+, SO42-), sodium (Na+) and chloride (Cl-) ions represent about 91% of all the seawater ions. Freshwater has much lower levels of salt ions.2
Salinity is often derived from electrical conductivity (EC) measurement. EC is measured by passing an electric current between two metal plates or electrodes in the water sample and measuring how readily current flows between the plates. The use of EC measurements to estimate the ionic content of seawater led to the development of Practical Salinity Scale 1978 (PSS-78).3
The PSS-78 has been considered by the Joint Panel on Oceanographic Tables and Standards and recommended by all oceanographic organizations as the scale in which to report future salinity data. The practical salinity of a sample of seawater is defined in terms of the ratio of the electrical conductivity of the seawater sample at the temperature of 15ºC and the pressure of 1 standard atmosphere, to that of a potassium chloride (KCl) solution containing a mass of 32.4356 g KCl in a mass of 1 kg of solution at the same temperature and pressure. A ratio equal to 1 corresponds to a practical salinity 35 (standard seawater).4 As the definition is a ratio, practical salinity is expressed as dimensionless number.
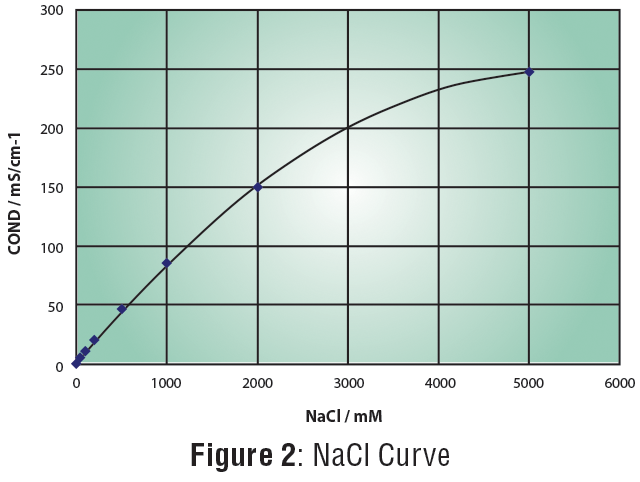
The LAQUAtwin Salt 11 pocket meter measures the conductivity value of a sample then converts it to salinity value based on the salinity standard curve selected. The sensor has two titanium metals coated with platinum black that resist corrosion and temperature sensor for accurate measurement. The meter is programmed with two standard calibration curves—seawater and sodium chloride (NaCl). The former follows the PSS-78 equation while the latter follows the equation in Figure 2.
Calibrate the LAQUAtwin Salt 11 pocket meter according to manufacturer’s instructions using the 0.5% (5ppt) and 5.0% (50ppt) NaCl standard solutions included in the kit.
Prior to calibration, make sure to select the standard calibration curve (either NaCl or Seawater) and the unit (either ppt or %) depending on your application and reporting unit requirement. If both seawater curve and ppt unit are selected, calibrate the meter with 5ppt NaCl standard. It is best to use 5ppt and 50ppt seawater standards, if available. Note that if ppt is set as the measurement unit, the meter will display only the salinity reading without the unit (dimensionless).
Place drops of water onto the sensor using the pipette included in the kit. Make sure that sample well is completely filled-up with sample and that there are no bubbles formed. Record the salinity reading once it is stable.
Before testing another sample, rinse the sensor with DI (distilled or deionized) water or with the next sample to be tested and dab a soft tissue to remove remaining water trapped inside the sample well.
Refer to Technical Tip 3 for detailed information on conditioning, cleaning, and storing the salt sensor. The technical tip can be viewed and downloaded from the support section of our website www.horiba-laqua.com.
Salinity is an important measurement in seawater or in estuaries where freshwater from rivers or streams mixes with salty ocean water since aquatic organisms have varying abilities to survive and thrive at different salinity levels. Saltwater organisms survive in salinity levels up to 40 ppt, yet many freshwater organisms cannot live in salinity levels above 1 ppt.2
Salinity affects the dissolved oxygen levels in water. The solubility of oxygen in water decreases as salinity increases. The solubility of oxygen in seawater is about 20% less than it is in fresh water at the same temperature.5
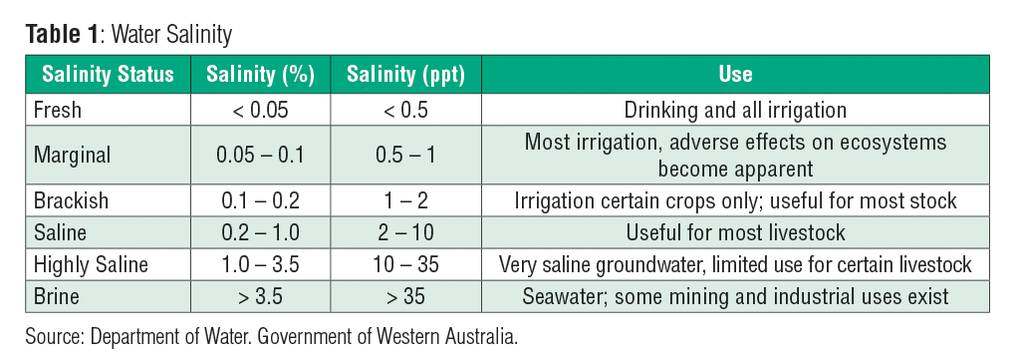
The table below shows the salinity values of different types of water and their uses.
Chapter 14 of the Volunteer Estuary Monitoring Manual, A Methods Manual, Second Edition, EPA-842-B-06-003. www.epa.gov/sites/production/files/2015-09/documents/2009_03_13_estuaries_monitor_chap14.pdf
Chloride and Salinity. www.ruf.rice.edu/~cbensa/Salinity/
Salinity. Wikipedia. en.wikipedia.org/wiki/Salinity
Practical Salinity Scale – 1978. Salinometry. salinometry.com/pss-78/
Salinity. Nooa Ocean Service Education. oceanservice.noaa.gov/education/kits/estuaries/media/supp_estuar10c_salinity.html
Revision 1.0, 28 October 2016
Você tem alguma dúvida ou solicitação? Utilize este formulário para entrar em contato com nossos especialistas.