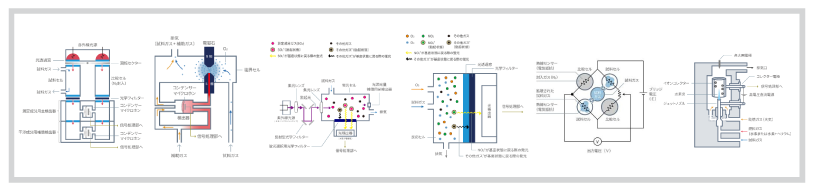
Figure 5: Structure and operating principle of electrochemical cell method galvanic cell type of oxygen analyzer
Measuring principle, structure and operating principle (Figure 5)
When a metal that is soluble in an electrolyte (base metal as the positive electrode of the cell) and a metal that is insoluble (precious metal as the negative electrode of the cell) are immersed in the electrolyte, the metal dissolves at the positive electrode and releases electrons, which reach the negative electrode. At the negative electode, the oxygen permeated through the thin film takes in electrons. (galvanic cell)
Reaction at positive electrode and negative electrode
Positive electrode
2Pb + 4OH- → 2PbO + 2H2O + 4e-
Negative electode
O2 + 2H2O + 4e- → 4OH-
Since the current by this electron flow is proportional to the oxygen permeated through the thin film, the oxygen concentration can be obtained by measuring this current.
Figure 5 shows a typical galvanic cell type oxygen analyzer that uses potassium hydroxide (KOH) as the electrolyte, lead(Pb) as the positive electrode, silver(Ag) as the negative electode, and fluorine-resin film as the thin film that permeats oxygen.