Quality Slide Program (QSP 2.0)
Accreditation Support
Qualification tool for cytology staff in accordance with standard NF EN ISO 15189
The QSP 2.0 software is a tool for high-definition imaging, didactic and very intuitive. It offers to the laboratory staff the examination of blood films, which are scanned and appraised beforehand.
It allows the laboratory to assess the ability of prospective examiners to correctly identify individual WBC populations and other identifiable elements.
One of the essential steps in accreditation is the assessment of staff skills in accordance with Chapters 5.1.2.d (Assessment of Competencies) and 5.1.3 (Continuous Training) of the NF EN ISO 15189 standard.
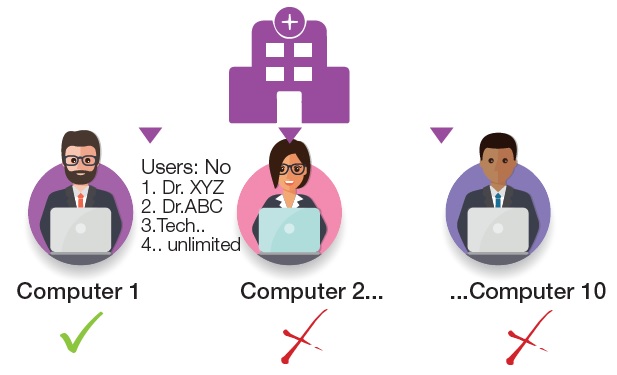
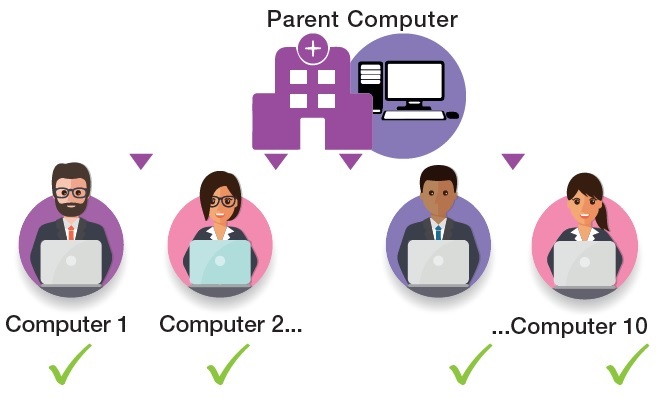
To meet this requirement, HORIBA Medical has specifically developed for the cytology benchtop an innovative solution, accessible to all laboratories, to control the slide review.
QSP 2.0 helps to standardise an area of the laboratory where results may sometimes be subjective. Continuous improvements to FBC technology over the years has led to low blood film review rates, however this has resulted in the reduction of experience, staff confidence and subsequently a skills gap in haematology morphology review. QSP 2.0 helps to overcome these challenges by improving training in blood film morphology and providing a means of assessing staff competencies. The QSP 2.0 is available now for both demonstration and lunchtime presentations.
Are you looking for the QSP Monthly Newsletter? ►Click here to go the QSP Newsletter page
*This product may not be available in your country or region at this time. Please contact your HORIBA Medical sales representative or distributor for more information.