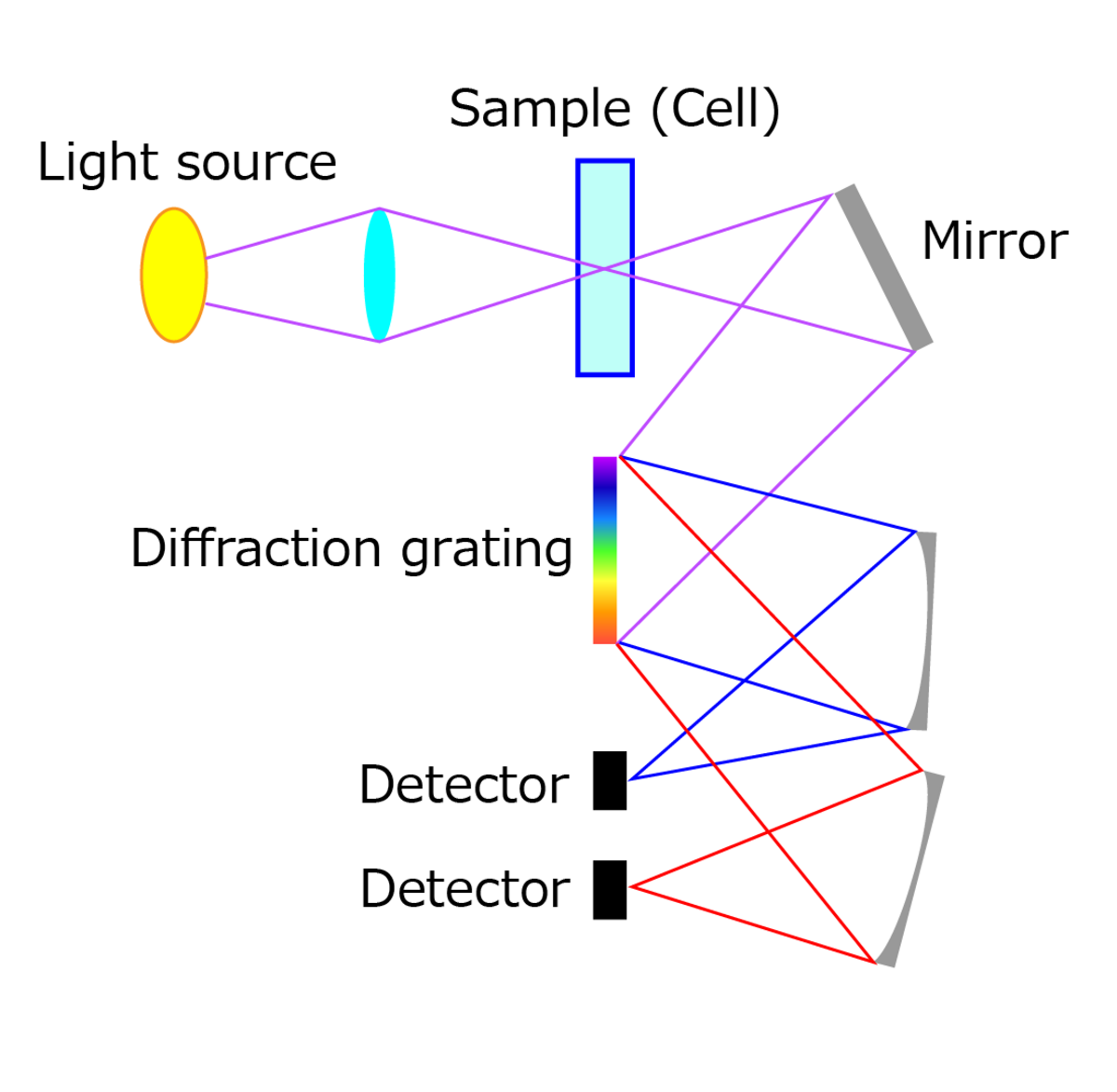
Absorption spectroscopy is a method of detecting concentration by measuring how much light is absorbed as it passes through a sample.
First, light from a light source is shone onto a cell with the sample to be measured. A mirror reflects light passing through the sample and transmits it to an optical component called a diffraction grating. The diffraction grating has the ability to separate the light beam into ultraviolet, near-infrared, and other individual wavelengths, before reflecting them at different angles. Each decomposed wavelength is directed to its own detector, which quantifies how much of that wavelength was absorbed by the sample.
To determine a sample’s concentration, samples with known concentrations are prepared and measured beforehand. Different types of these samples are measured to collect data on how much light of each wavelength is absorbed at each concentration. The collected data is then analyzed to create a calibration curve that represents the relationship between the sample’s concentration and light absorption.
This calibration curve is used to convert the amount of light absorbed to concentration, enabling detection of the sample’s concentration.
Chemical Solution Monitoring System
Non-Contact Chemical Concentration Monitor
Optical Fiber Type Hot Phosphoric Acid Concentration Monitor
Fiber Optic Type Chemical Concentration Monitor
Fiber Optic Type Chemical Solution Concentration Monitor
High Precision, High Stability Chemical Concentration Monitor
Stand-alone Type Chemical Concentration Monitor