Measurement of ORP
Measurement of ORPs
As mentioned in “The Basis of ORP Measurement,” ORP is short for oxidation-reduction potential. The ORP of sample water is determined by the chemical equilibrium of the oxidizing and reducing substances in it.
When the oxidizing substance is Oxn+, the reducing substance is Red, and the exchanged electron is ne-, the relationship among them and the ORP can be expressed as follows:
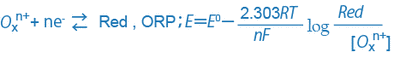
Generally, sample water contains different kinds of oxidizing and reducing substances, and the ion species cannot be identified by the ORP alone. However, if the sample water contains a dominant oxidation/reduction system, the ORP provides a clue about the ratio between the oxidizing substances and reducing substances.
Chemical oxygen demand (COD) is an application of the measurement of ORPs. COD is an indicator of the presence of organic substances in wastewater.
COD indicates the degree of organic pollution of wastewater as the amount of an oxidant (potassium permanganate or KMnO4 is generally used as the oxidant) required to chemically oxidize organic substances in the wastewater. The COD of wastewater is found by titrating a certain amount of the wastewater with a solution of KMnO4. The endpoint of titration is detected by the slight coloration caused by KMnO4 or the ORP. In many cases, wastewater is colored or turbid to begin with, and the measurement of the ORP provides an effective means of detecting the endpoint of titration (this is called “potentiometric titration”).
For example, when oxalic acid or (COOH)2, which is an organic substance, is acidified with sulfuric acid and then titrated with potassium permanganate, the following reaction occurs:
![]() This chemical equation means that oxalic acid, which is an organic substance, is oxidized by potassium permanganate and decomposed into carbon dioxide and water. The amount of potassium permanganate required by this reaction is the COD, and a rapid change in ORP occurs at the endpoint of titration.
This chemical equation means that oxalic acid, which is an organic substance, is oxidized by potassium permanganate and decomposed into carbon dioxide and water. The amount of potassium permanganate required by this reaction is the COD, and a rapid change in ORP occurs at the endpoint of titration.
When measuring the ORP, switch the pH meter to the mV range, connect the metal electrode to the G terminal and the reference electrode to the R terminal (Platinum, gold, or other noble metals are used as the metal electrode. Platinum electrodes are especially popular). As shown in the expressions of oxidation-reduction potentials given before, ORPs are also affected by the temperature (T), so it is important to keep the temperature constant (for example, at 25ºC) when measuring the ORP.
In order to check whether an ORP meter is functioning properly, a solution with a known ORP must be prepared. We sell powders that use the principle of a quinhydrone electrode to prepare a standard ORP solution. The electric potential of quinhydrone (a quinine-hydroquinone system) is determined by its pH. Our powders use this nature of quinhydrone. Dissolve the powder in a specified volume of pure water (ion-exchanged water), and check that the mV meter with a metal electrode and a reference electrode connected as mentioned above indicates an ORP within the specified range in this solution (switch the pH meter to the mV range).
Powders for ORP standard solution
Model: 160-51
(neutral phosphate + quinhydrone: dissolve in 250 ml of pure water.), ORP 89 ± 15 mV
Model: 160-22
(phthalate + quinhydrone: dissolve in 250 ml of pure water), ORP 258 ± 15 mV
Both models assume that ORPs are measured with an Ag/AgCl reference electrode at 25ºC.
(Phthalate + quinhydrone; constant volume 250mL), ORP 258±15mⅤ
Both vs.Ag/AgCl at 25℃