Foods, Medicines, Cosmetics and Medical care
Food, Brewing and Fermentation

The food and beverage products that most require pH measurement are those whose production involves enzyme reactions, such as bread, liquor, beer, soy sauce, miso, cheese and milk products. This is because the action of enzymes is affected by pH, and each has an optimum pH critical to the results.
With some foods, pH is an important factor in taste and safety.
Each food has an optimum pH value, and if the pH value is too high or too low, it is likely that it won't taste right or that safety has been compromised by some foreign substance. Every maker of processed food checks pH as part of the effort to make good-tasting food of consistent quality.
Medicines and Cosmetics

In the pharmaceutical and cosmetics industries, pH is measured in order to check chemical reactions in production. Speed of reactions depends on the pH of a solution, and the end point of a reaction can be estimated by knowing the pH.
For example, in the production of antibiotics, pH must be controlled in the fermentation processes to maintain high yield and antibacterial properties.
Also, as medicines and cosmetics are taken into the human body or applied to human skin, the strictest quality control is required.
If there is big difference in pH between skin and cosmetic products, the results could be disastrous. If a medicine is of the wrong pH, that medicine could be a poison.
Medical Organizations

Medical organizations measure pH to adjust reagents. They also measure the pH of such body fluids as blood, gastric juices and urine for medical research, medical testing and treatment.
Also, researchers at dental colleges measure pH when studying tooth decay. They have found that pH in the mouth begins to decrease five minutes after eating and that the fluid in the mouth begins to dissolve tooth enamel when pH reaches less than 2.5.
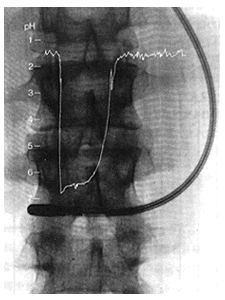
Electrodes in the Stomach
Ultrasmall compound pH electrodes can be inserted into the digestive tract to check pH of gastric juices in active status.
The Living Body and pH
Blood (Healthy person) 7.40 (7.36~7.44)
Cerebral Fluid 7.32
Gastric Juices 1.5-8.5
Pancreatic Juices 8.6-8.8
Saliva 7.46 (7.2-7.6)
Semen 7.4 (7.1-7.6)
Perspiration 3.8-6.5
Urine 4.0-7.8
[Reference] Electrolyte Abnormality, Internal Medicine Mook,* No. 27 (1985), Editor/Planner: Shozo Koshikawa: pH Regulation System, p. 116, (1985), Author: Kunio Suwa
The movie described below, shows the importance of blood pH in an easily understandable manner:
The Andromeda Strain (an SF novel published in 1969 and filmed in 1971) marked a breakthrough in the career of American novelist Michael Crichton, who later became famous for Jurassic Park. The premise of the novel is that a recovered satellite has brought back a pathogen which grows in human blood, killing the infected person in the process, albeit only within the normal blood pH range (a narrow range around 7.40). In the movie, a crying baby (in a state of alkalosis, in which its blood pH is more alkaline than the normal level due to the excessive discharge of CO2 caused by hyperventilation) and a drunken old man (in a state of acidosis, in which excessive alcohol intake has caused his blood pH to be more acidic than normal) survive. This SF movie cleverly utilizes the narrow normal range of blood pH.
First | Next page » Industrial fields