


If you have been looking for a pH meter, you have probably seen the words “Automatic Temperature Compensation” or ATC in the specifications or advertisements of various pH meter brands and wondered what it means. This technical tip answers the three frequently asked questions about ATC in pH measurement.
Since temperature affects pH, the actual temperatures of pH buffers and samples must be measured and take into account in calibration and measurement.
A 3-in-1 pH electrode (combination pH electrode with built-in temperature sensor) or a combination pH electrode together with a separate temperature probe is recommended to be used with the pH meter. When immersed in solution, the temperature sensor or probe measures the actual temperature, which is then transmitted directly to the pH meter.
Click image to enlarge.
Figure 1: Examples of 3-in-1 pH electrode and combination pH electrode and separate temperature probe
During calibration, the pH meter automatically corrects the reading of the pH buffer in use to the expected value at the actual temperature.
Prior to measurement, pH calibration must be performed with the pH meter and pH electrode using at least two pH buffers that bracket the pH values of samples. HORIBA pH meters are programmed with the expected pH values of commonly used sets of pH buffers (e.g., USA, NIST, DIN) at different temperatures. For the pH meter to recognize and calibrate the pH buffers correctly, the appropriate pH buffer group must be selected and set in the set-up mode of the pH meter. The use of pH buffers not matching the pH buffer group setting results in error code on the pH meter or inaccurate calibration leading to unreliable measurements.
Click image to enlarge.
During calibration, the pH meter detects the temperature measured by the temperature sensor or probe as well as the potential (mV) measured by the pH electrode. The pH meter then recognizes the pH buffer in use based on the measured potential and assigns the correct value of the pH buffer based on the measured temperature. To verify whether the pH meter has calibrated the correct value of the pH buffer at the measured temperature, refer to the pH vs temperature table on the label of the pH buffer bottle.
Click image to enlarge.
Figure 2: Screenshots of F-74 meter before, during, and after calibration with pH 7 buffer at 25°C
Temperature also affects the pH electrode slope.
The pH electrode behaviour follows the Nernst equation: E = E0 + 2.303 (RT/nF) log aH+ where slope, also called sensitivity, is denoted by -2.303 RT/nF and pH is equal to -log aH+. Since R, F, and n are constants, the slope changes with temperature and this effect can be compensated by a pH meter with ATC capability.
Click image to enlarge.
Figure 3: Nernst equation
Click image to enlarge.
Figure 4: Theoretical slopes of an ideal pH electrode at different temperatures
After calibration, the pH meter generates slope at the measured temperature according to the Nernst equation. HORIBA pH meters report slope for every two consecutive calibration points in percentage (% = actual / theoretical slope x 100). The slope should be within the range 90-105%.
Figure 5: Example of pH calibration result
During measurement, the pH meter applies the slope to calculate the pH values of samples.
To obtain accurate results, samples should be at the same (or as close as possible) temperature as the pH buffers used in calibration. This can be achieved by allowing the pH buffers and samples to equilibrate at room temperature or by placing them in a water bath with temperature control.
In this case, the pH meter shows the default temperature 25°C. It is more convenient to use a 3-in-1 pH electrode because the measured temperature is automatically transmitted to the pH meter and only one electrode is immersed in each solution. However, if a 3-in-1 pH electrode or separate temperature probe is not available, you may manually enter the temperatures of your pH buffers and samples into the pH meter. To do this, measure the temperatures using a calibrated thermometer or other temperature measuring device and then enter the temperatures into the manual temperature compensation (MTC) setting of the pH meter before doing calibration and measurement. Refer to the meter instruction manual for the location of the MTC setting in the set-up mode and the procedure for entering the temperature.
HORIBA pH meters have ATC and MTC settings. The ATC is used to correct the temperature reading of the temperature sensor or probe connected to the meter while the MTC is used to enter the temperature reading of a separate temperature measuring device into the meter.
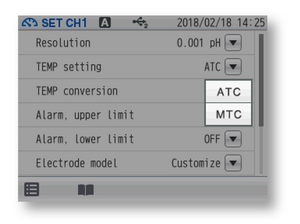
Figure 6: ATC and MTC settings in F-74 meter
No. Just like pH buffers, the chemical compositions of samples may vary and therefore samples may react differently to temperature. Also, laboratory pH meters are typically not programmed to correct the pH values of samples at the actual or other temperature. As such, it is important to record the pH value together with the temperature after measuring a sample.
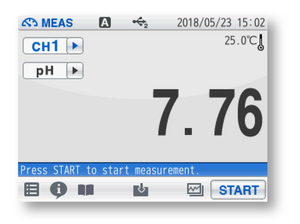
Figure 7: Record the pH and temperature of each sample
HORIBA F-70 series colour touchscreen pH bench meters have temperature conversion feature, which can be utilized if the temperature coefficient of a sample is known.
23 May 2018, Rev. 0
Tiene alguna pregunta o solicitud? Utilice este formulario para ponerse en contacto con nuestros especialistas.
HORIBA Advanced Techno, Co., Ltd.
31, Miyanonishi-cho, Kisshoin
Minami-ku Kyoto 601-8306 Japan
Tel: +(81) 75 321 7184
Fax: +(81) 75 321 7291
HORIBA Instruments (Singapore) Pte Ltd.
83 Science Park Drive, #02-02A,
The Curie 118258 Singapore
Tel: +65 6 908 9660
Fax: +65 6 745 8155
Mail: laqua(at)horiba.com
HORIBA UK Limited Northampton Office
Kyoto Close,Summerhouse Road,
Moulton Park
Northampton NN3 6FL UK
Tel: +(44) 1604 542 600
Fax: +(44) 1604 542 699
Mail: waterquality(at)horiba.com
HORIBA Instruments Incorporated Head Office
9755 Research Drive
Irvine California 92618 USA
Tel: +1 800 446 7422
Fax: +1 949 468 1790
Mail: labinfo(at)horiba.com
You might also like to know...