After analyzing the constituent elements and quantities using the X-ray fluorescence analyzer, the Ryugu samples then will be investigated using a Raman spectrometer. More specifically, Raman micoanalysis sheds light on the bonding of the elements in the sample. For example, even though X-ray fluorescence analysis is able to find the presence of the elements oxygen (O) and iron (Fe), it is unable to determine whether these are bonded in the form of Fe2O3 (iron(III) oxide) or Fe3O4 (iron(II,III) oxide), or some other combination. Although these two forms of iron oxide known respectively as hematite and magnetite are made of the same elements, they are completely different minerals.
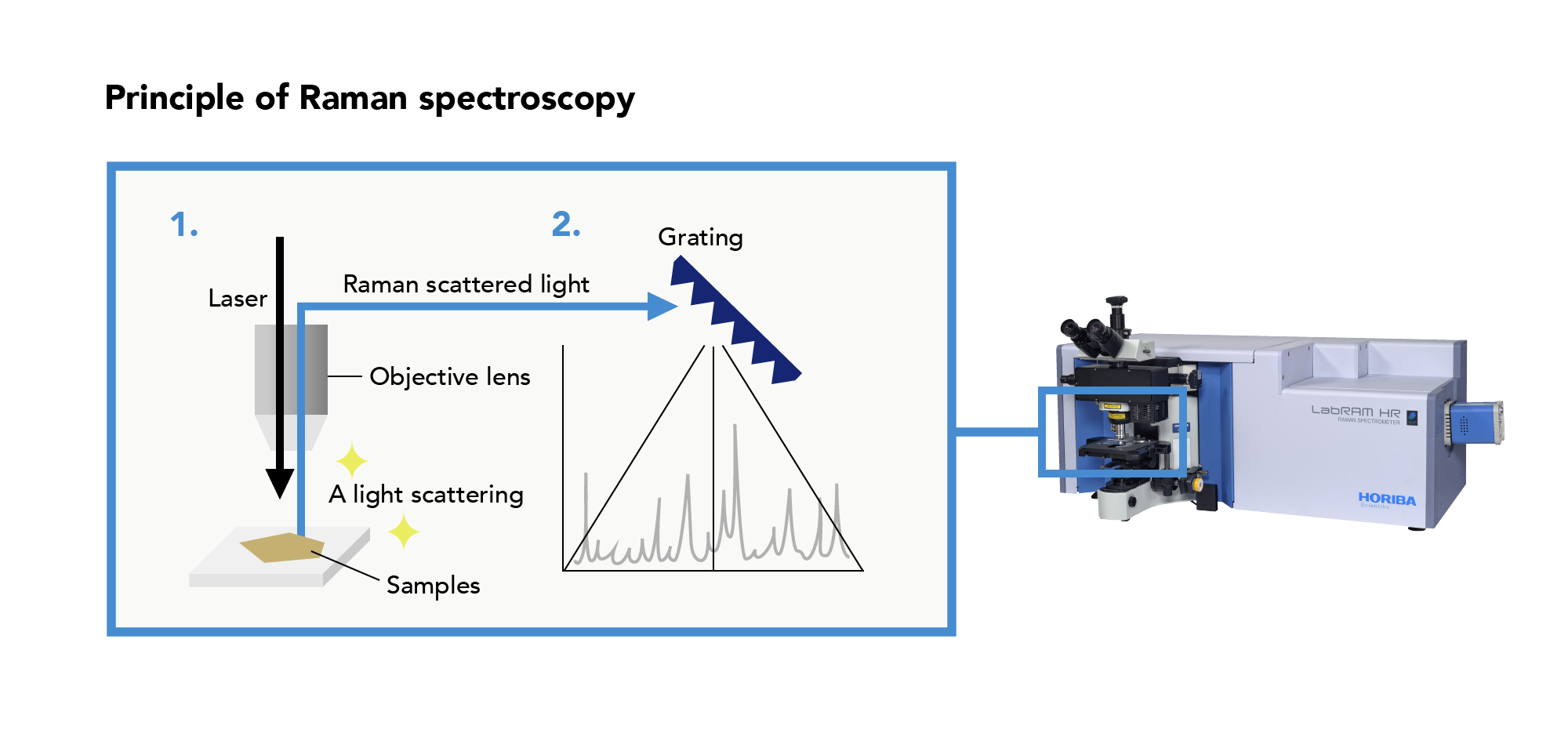
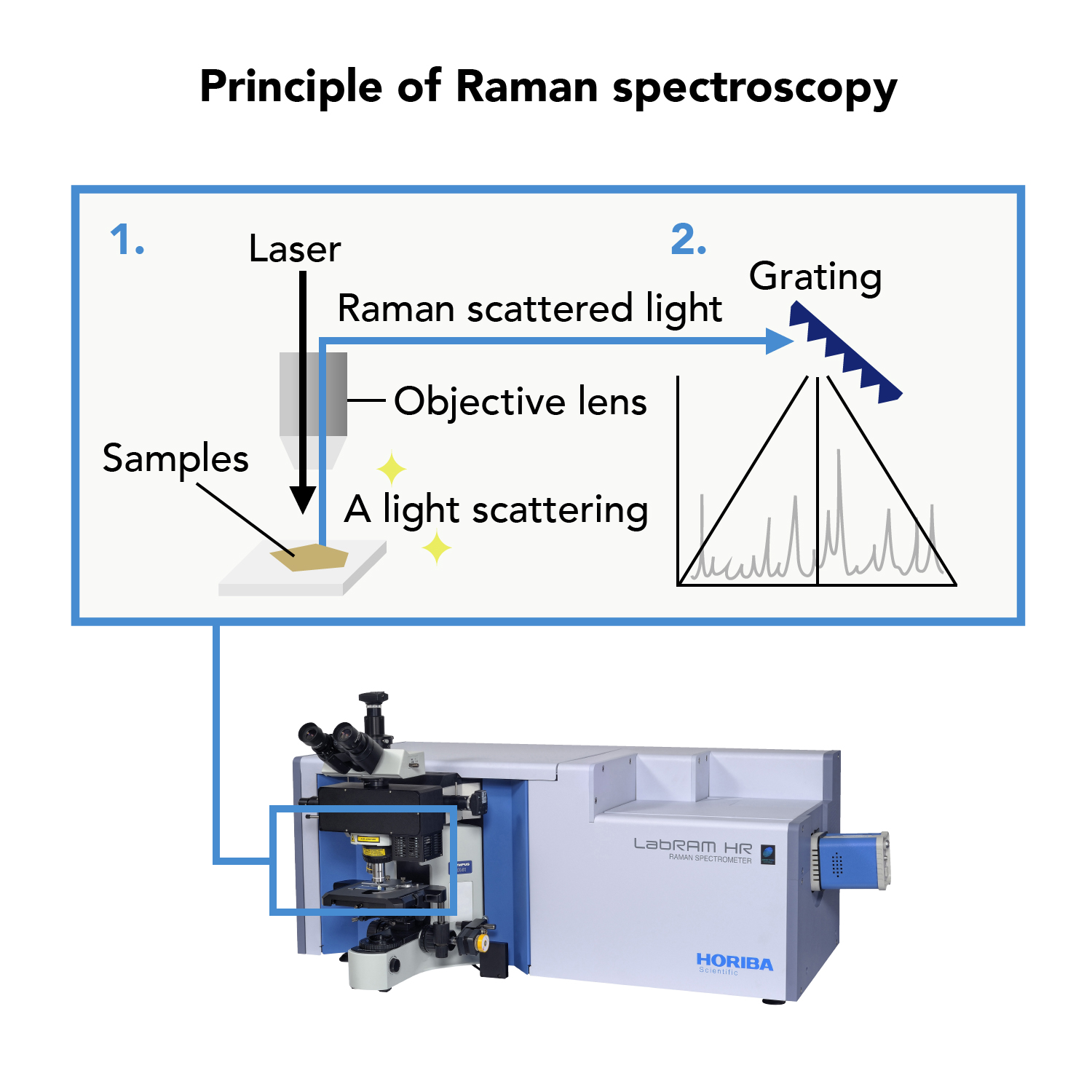
A Raman spectrometer can identify a mineral by reading differences in molecular structure from a Raman spectrum.
Raman spectroscopy is a technique of analysis that uses light to evaluate materials. When a material is irradiated with light, light of a different wavelength, known as “Raman scattered light,” is emitted by the material. Taking advantage of the fact that the wavelength and intensity of “Raman scattered light” is unique to a particular material, Raman spectroscopy is used to determine the composition, molecular structure, stress, and other characteristics of materials.
Raman spectroscopy was named after the discovery of Raman scattering by Indian physicist C. V. Raman in 1928.
Ryugu is believed to be a C-type asteroid, which means it is thought to contain water and organic matter, yet still retains primitive information from the early days of the solar system. This kind of analysis could lead to the discovery of previously unimagined compounds or minerals that might hold the key to the origin of life on Earth.

Raman spectrometer
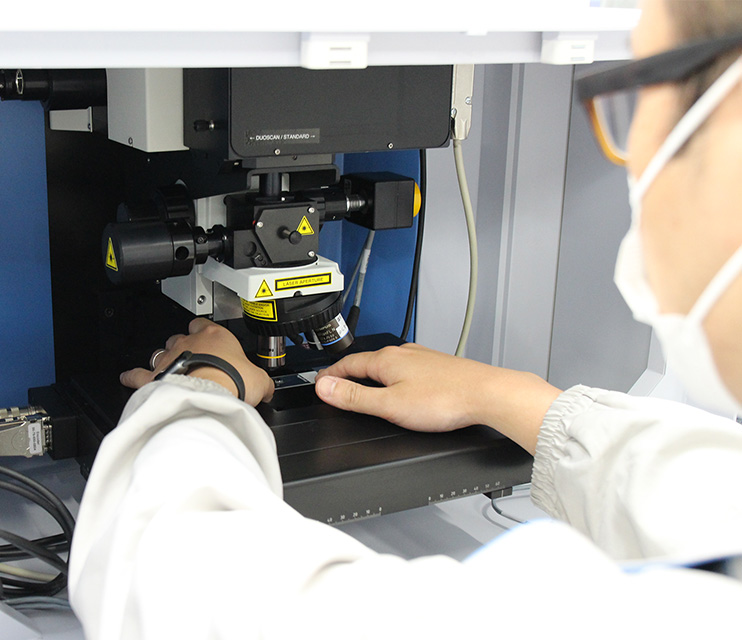
Identifying minerals using a Raman spectrometer