Titration Method
Winkler’s Method
Add a manganese peroxide solution and a sodium hydroxide solution to sample water, and a precipitate of manganese hydroxide (II) will be produced. This precipitate of manganese hydroxide (II) reacts with dissolved oxygen in the water and is oxidized according to the amount of DO, forming a brown precipitate.
If DO is not present:
![]()
If it reacts with DO:
![]()
Dissolve this brown precipitate in an acid in the presence of iodine ions (I-), and iodine (I2) will be released according to the amount of DO. Then, titrate the released iodine (I2) with sodium thiosulfate and determine the quantity.
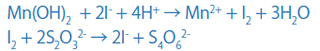
Modified Winkler’s Method Using Sodium Azide
This method is intended to improve the accuracy of DO measurement by Winkler’s method. In this method, I2 remaining after the titration of I2 with sodium thiosulfate in the final process of Winkler’s method is titrated again with a starch solution.
Another titration method is Miller’s method.
Next page Diaphragm Electrode Method