What are ions?

Before discussing the measurement of ions using ion selective electrodes, let us talk about ions in aqueous solution.
“Concentration of Hydrogen Ions” in “The Basis of pH ” section of this site states that, “Water is represented by the molecular formula H2O, and is mostly in the form of H2O molecules, which are very stable. However, a small proportion of water molecules separate into hydrogen ions H+ and hydroxyl-ions OH-.”
Because water is in this form at room temperature, it dissolves many kinds of substances. (However, some substances do not dissolve in water, as would be expected from the Japanese phrase “water and oil” used to indicate things that do not mix because of their fundamentally different natures.) Let us look at the differences between the properties of aqueous solutions of sugar and salt.
When sugar, particularly high-purity granulated sugar, is dissolved in water, the ease with which electricity (or current, to be more precise) flows through it is almost the same as that of the original water. In contrast, current flows extremely easily through salt solution. What is the reason for this difference?
It is that sugar (C6H12O6) does not turn into ions, whereas salt (NaCl) completely ionizes into sodium ions (Na+) and chloride ions (Cl-), allowing electric current to flow. We will not go into the detailed reasons for this behavior here; it is sufficient to state that sodium atoms (Na), due to their nature, tend to release an electron e- to become Na+ sodium ions, and chlorine atoms (Cl) tend to accept an electron e- to become Cl- chloride ions. Na+ and Cl- electrically attract each other to form the compound known as salt (NaCl). A substance that is formed like this is called an ionic compound. Meanwhile, the rather complicated compound known as sugar is formed by carbon, hydrogen, and oxygen atoms combining by sharing some of their electrons (valence electrons), i.e. it is a covalent compound. Even after dissolving in water, sugar molecules retain their form and do not turn into ions. Although sugar and salt seemingly dissolve in water in the same manner, they are greatly different in their ionization/non-ionization behavior in water.
The following illustrates these points:
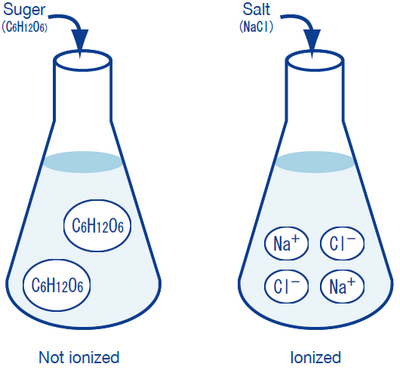

Sodium ions Na+, potassium ions K+, chloride ions Cl-, and hydrogen-carbonate ions HCO3- are present in human blood and lymph fluid, and play important physiological roles, such as controlling osmotic pressure. These ions are known as electrolytes, a technical term used in medicine and physiology. (In physics and chemistry, “electrolyte” is a more general term which, briefly stated, can be said to indicate any type of ion.)
An ion carrying a positive charge is called a cation, and one carrying a negative charge is called an anion. The number of electrons that a compound or atom accepts or releases when it becomes an ion is called its valency, and valencies of one, two and three are described respectively by the terms univalent (or monovalent), divalent (or bivalent), and trivalent. For example, Na+ is a monovalent cation, Cl- is a monovalent anion, Ca2+ is a divalent cation, SO42- is a divalent anion, Fe3+ is a trivalent cation, and PO43- is a trivalent anion. Ions of larger valency also exist. Water always contains the same number of anions and cations, and looks electrically neutral when viewed from outside.
We hope that the above explanation has helped your understanding of what ions are.
The next section describes ion electrodes, which were developed for the purpose of easily measuring ions as they are, in their original ionic state.
Relation page The Basis of Ion