

Polyelectrolytes are polymer chains with an electrolyte group on every repeat unit. These polymers are charged when dissolved in a polar solvent due to dissociation of small counterions that leave behind a charged macro ion. In addition to synthetic polymers, the topic of this note, polyeletrolytes include biologically important molecules such as polypeptides and DNA.
The charge on a polyelectrolyte is a function of the solution conditions. Unlike the case of simple electrolytes, not all counterions will dissociate away from a polyelectrolyte. As the magnitude of the charge on the chain increases, it becomes progressively more difficult to remove the next ion. This phenomenon is known as counterion condensation.
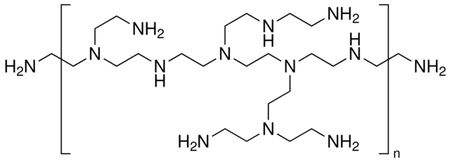
Figure 1: Illustration of structure of branched PEI. The NH groups give the polymer a charge that is manipulated via, among other things, solution pH.
Polyelectrolyte behavior is different from that of uncharged polymers. For example, due to electrostatic repulsion between different segments on the same chain, a polyelectrolyte chain will tend to be more rod-like than a typical Gaussian chain. Furthermore, due to long range electrostatic interactions, polyelectrolyte solution properties are different from those of neutral polymers. For example, the second virial coefficient (a measure of solution thermodynamics) can be an order of magnitude higher for a polyelectrolyte than a neutral polymer. These effects will lead to significant changes in solution viscosity.
Due to these solution effects polyelectrolytes have a number of applications. They are used as viscosity modifiers. Furthermore, due to their charge, they are used to modify the surface charge of nanoparticles to either stabilize suspensions by increasing particle surface charge or to initiate flocculation by a) neutralizing surface charge and b) acting as a bridge between particles.
Polyethylenimine (PEI) is a cationic polyelectrolyte. The structure is given in Figure 1 below. PEI is used to attach negatively charged cells in biology. It is also used for DNA transfection (introducing new DNA into cells). PEI can also be used to capture carbon dioxide. Much of the useful behavior of PEI is obtained by its charged state and the ability to manipulate it using pH.
Since they are large molecules, polyelectrolytes scatter light in much the same way as particles. Due to their charge, they respond like particles to an applied electric field. Thus, one can measure polyelectrolyte mobility and extract a zeta potential value to characterize these materials.
Branched PEI was obtained from Sigma Aldrich as a 50 weight percent solution in water. This was then further diluted with 1 mM aqueous KCl to prepare solutions with appropriate polymer concentration. The small amount of KCl is used as a background electrolyte to ensure that the effect of small quanitites of impurities do not dramatically affect zeta pottential results. The polymer molecular weight was given by the manufacturer as 750,000 g/mol by light scattering.
Zeta potential was then measured with the SZ-100V2 Nanoparticle Analyzer, shown in Figure 2. Measurements were repeated six times.

Figure 2: SZ-100V2 Nanoparticle Analyzer
The obtained zeta potential values are shown in Table 1 below. Figure 3 shows the effect of concentration on zeta potential. As expected, zeta potential decreases with increasing ionic strength due to the increasing concentration of macro-ions and increasing chain overlap.
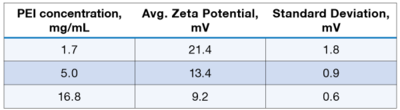
Table 1: Zeta potential of PEI as a function of polymer concentration
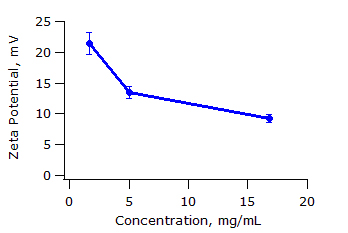
Figure 3: Zeta potential of PEI as a function of polymer concentration. Error bars correspond to one stadard deviation.
The SZ-100V2 can be used to characterize the charge of polymeric species. Zeta potential decreases as polyelectrolyte concentration is increased due to the increasing ionic strength of the suspension and intermolecular interactions. The zeta potential of charged polymers such as polyelectrolytes can easily be analyzed with the SZ-100V2.
Nanoparticle Analyzer
У вас есть вопросы или пожелания? Используйте эту форму, чтобы связаться с нашими специалистами.