Raman spectroscopy, combined with advanced chemometric modeling, is a rapid, non-invasive, and non-destructive technique for comprehensively characterizing biological products. By generating unique molecular fingerprints, it allows manufacturers to monitor protein stability, metabolic shifts in bioreactors, and trace contaminants with high sensitivity and minimal sample preparation.
BioProcess International recently published an article on this topic titled 'Streamlining Biopharmaceutical Development and Manufacturing: A Detailed Exploration of Raman Spectroscopy. In it, you will learn how Raman spectroscopy, by utilizing inelastic light scattering, offers deep molecular-level insights into protein tertiary structures and cellular metabolism. The article demonstrates the effectiveness of Raman in providing real-time feedback during production, ensuring product integrity, and accelerating batch-release timelines through technologies like Surface-Enhanced Raman Spectroscopy (SERS).
Read the full story in BioProcess International here.
Raman spectroscopy relies on the inelastic scattering of light. When a laser interacts with a sample, a small fraction of photons undergoes a shift in energy that corresponds to the specific vibrational modes of the molecules. These shifts create "spectral fingerprints" that provide detailed information about the chemical structure and molecular composition of the sample.
Unlike infrared (IR) spectroscopy, Raman spectroscopy has a low sensitivity to water. This allows scientists to analyze proteins and cell cultures in their natural aqueous environments without the need for extensive sample preparation or dehydration, which could otherwise alter the biological state of the product.
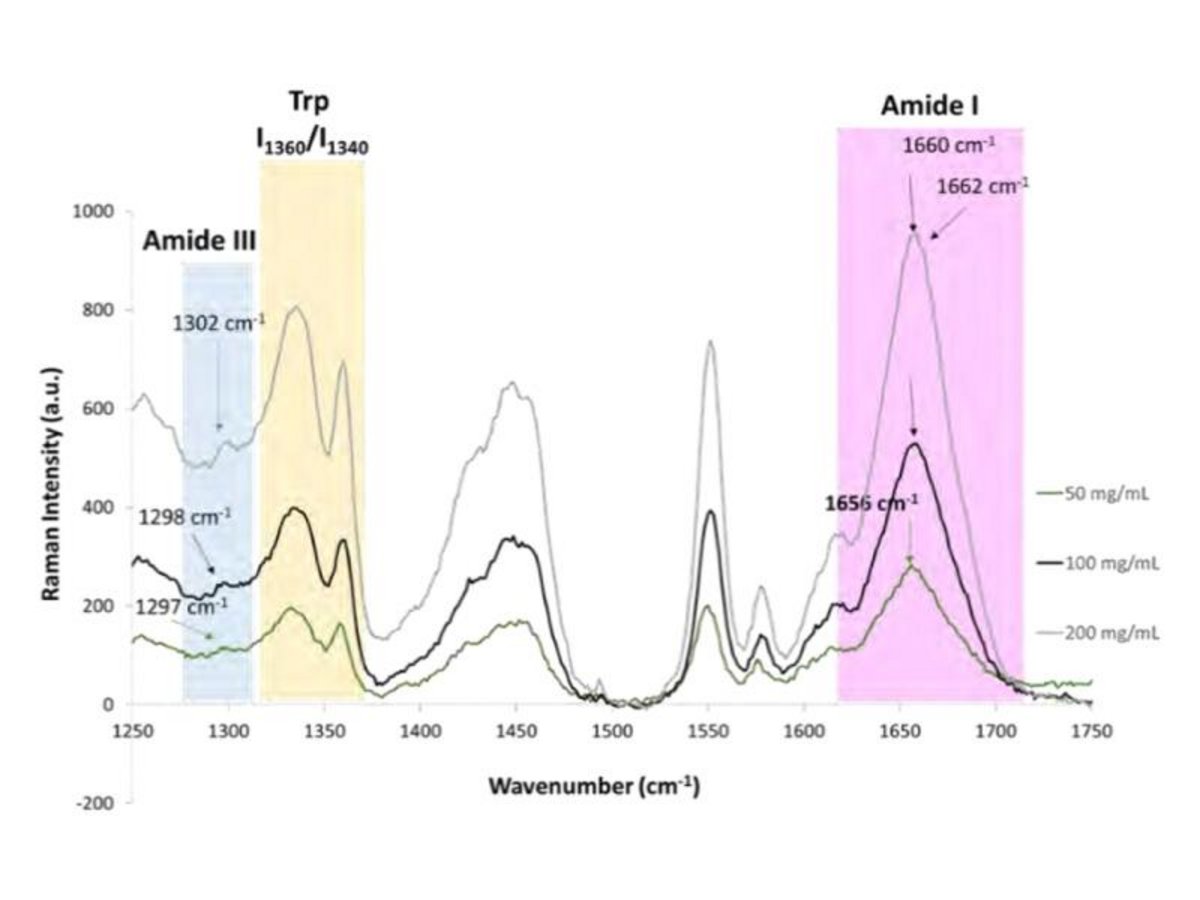
Beyond identifying basic secondary structures, Raman can pinpoint subtle conformational changes in tertiary structures. By using advanced algorithms for spectral deconvolution, researchers can monitor specific bands—such as aromatic side-chain vibrations or disulfide bonds. This allows for the calculation of thermal stability and the identification of denaturation points with a precision of ±1 °C.
Raman probes can be integrated directly into bioreactors to provide in situ monitoring of cellular metabolism. By using multivariate data analysis (like PLS-DA), the system can correlate spectral changes with critical quality attributes (CQAs) such as viable cell density, nutrient levels (glucose/lactate), and glycosylation patterns. This enables automated feedback loops that adjust feeding strategies in real time.
SERS is a specialized form of Raman spectroscopy used to detect trace contaminants at extremely low concentrations (parts per billion). It utilizes metallic nanoparticles (usually gold or silver) to amplify the Raman signal by factors of $10^6$ to $10^{10}$. This makes it possible to identify leachable impurities or toxins that are invisible to standard Raman techniques.
Because Raman datasets are highly complex, scientists use Artificial Intelligence (AI) and machine learning algorithms to extract predictive insights. These models can be trained to recognize patterns that correlate with process deviations, leading to a significant reduction in batch failures and improved process efficiency by merging Raman data with other parameters like pH and dissolved oxygen.
As Raman moves from the lab to the manufacturing floor, industry bodies are focusing on standardization and validation. This includes developing clear data-analysis protocols, robust spectral libraries, and ensuring data integrity to meet the stringent quality standards and regulatory mandates of the biopharmaceutical industry.
Do you have any questions or requests? Use this form to contact our specialists.