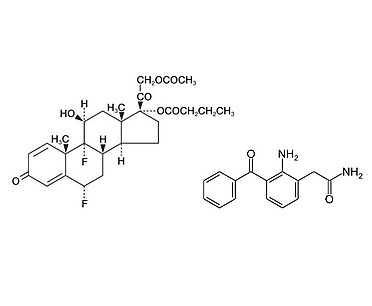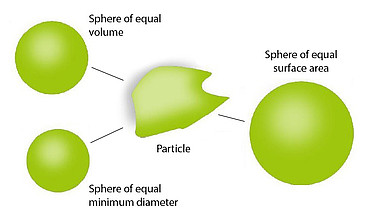
Small molecule therapeutics are very flexible, with a variety of administrations routes, from pills, capsules, to inhalation devices. The quality of many of these products depends on knowing how much of a component is present, how it is distributed, and what size it is. HORIBA has a portfolio of analytical instruments that address these questions, with a full range of particles sizing techniques, to best-in-class Raman microscopy. This portfolio of products speeds up product development, answers critical questions for troubleshooting, and is indispensable for contaminant identification.